135065-71-3
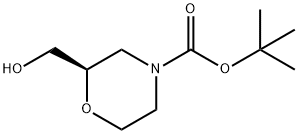 135065-71-3 结构式
135065-71-3 结构式
基本信息
(R)-N-BOC-2-吗啉甲醇
(R)-N-BOC-吗啉-2-甲醇
(R)-N-Boc-2-羟甲基吗啉
N-叔丁氧羰基-(R)-2-吗啉甲醇
(R)-N-BOC-2-羟基甲基吗啉
(R)-4-叔丁氧羰基-2-羟甲基吗啉
(R)-2-羟甲基吗啉-4-羧酸叔丁酯
(R)-2-羟甲基吗啉-4-甲酸叔丁酯
(R)-2-羟甲基吗啉-4-甲酸叔丁基酯
(R)-2-hydroxyMethyl-4-BOC-Morpholine
(R)-4-BOC-2-HYDROXYMETHYL-MORPHOLINE
(R)-N-Boc-2-hydroxymethylmorpholine,99%
(R)/(S)-N-Boc-2-hydroxymethylmorpholine
(R)-N-Boc-2-hydroxymethylmorpholine,99%e.e.
(R)-4-t-butoxycarbonyl-2-(hydroxymethyl)morpholine
Tert-Butyl(R)-2-(Hydroxymethyl)Morpholine-4-Carboxylate
(R)-N-Boc-2-Hydroxymethylmorpholine ISO 9001:2015 REACH
tert-butyl (2R)-2-(hydroxymethyl)morpholine-4-carboxylate
物理化学性质
制备方法
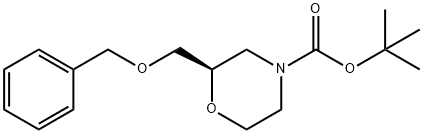
135097-68-6

135065-71-3
在氢气氛下,以10% Pd/C(1.98 g,1.86 mmol)为催化剂,将中间体111(19.8 g,64.4 mmol)搅拌反应16小时。反应完成后,通过真空过滤移除催化剂,滤液经减压浓缩,得到13.98 g(100%收率)的(R)-N-Boc-2-羟甲基吗啉,产物为无色粘性油状物,静置后结晶。产物的1H NMR(250 MHz,氯仿-d)数据如下:δ [ppm] 3.98 - 3.75(m,3H),3.73 - 3.41(m,4H),3.03 - 2.83(m,1H),2.82 - 2.65(m,1H),2.12(t,J = 5.9 Hz,1H),1.45(s,9H)。
参考文献:
[1] Heterocycles, 1993, vol. 35, # 1, p. 105 - 109
[2] Patent: WO2016/91776, 2016, A1. Location in patent: Page/Page column 290
[3] Patent: WO2008/124575, 2008, A1. Location in patent: Page/Page column 112; 113-114
[4] Patent: US2010/184805, 2010, A1. Location in patent: Page/Page column 30-31
[5] Patent: WO2007/70201, 2007, A1. Location in patent: Page/Page column 176
常见问题列表
(R)-N-Boc-2-羟甲基吗啉通过模板筛选用于制备Checkpoint激酶1的抑制剂。
