13679-70-4
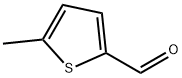 13679-70-4 结构式
13679-70-4 结构式
基本信息
5-甲基-2-噻吩基甲醛
5-甲基噻吩-2-甲醛
5-甲基噻酚-2-甲醛
5-甲基噻酚-2-甲醛, 98+%
5-METHYL-2-THIOPHENECARBOXALDEHYDE
5-METHYL-THIOPHEN-2-ALDEHYDE
5-METHYLTHIOPHENE-2-ALDEHYDE
5-METHYLTHIOPHENE-2-CARBALDEHYDE
5-METHYLTHIOPHENE-2-CARBOXALDEHYDE
AKOS B015983
AKOS BBS-00003245
FEMA 3209
LABOTEST-BB LT00936047
TIMTEC-BB SBB004249
2-Formyl-5-methylthiophene
2-Thiophenecarboxaldehyde, 5-methyl-
5-Methyl-2-formylthiophene
5-Methyl-2-thiophencarboxaldehyde
5-methyl-2-thiophenecarboxaldehyd
Thiophen-2-carboxaldehyde, 5-methyl
thiophene, 2-Methyl, 5-formyl
5-methyl-2-thiopene carboxaldehyde
5-METHYL-2-THIOPHENECARBOXALDEHYDE 98+%
物理化学性质
安全数据
制备方法
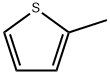
554-14-3

68-12-2

13679-70-4
一般步骤:在氮气保护下,将0.99 M的乙基氯化镁(EtMgCl,0.61 mL,0.6 mmol)的四氢呋喃(THF)溶液冷却至0°C,缓慢滴加二环己胺(0.01 mL,0.05 mmol)和2-甲基噻吩(1a,0.048 mL,0.50 mmol)。将反应混合物升温至60°C并搅拌24小时。随后,依次加入1.4 mL THF和N,N-二甲基甲酰胺(DMF,0.5 mL,6.46 mmol),继续在60°C下搅拌1小时。反应完成后,用饱和氯化铵水溶液(1.0 mL)淬灭反应,将混合物倒入乙醚/水的混合溶剂中,分离有机相和水相。水相用乙醚萃取两次,合并有机层并用无水硫酸钠干燥。减压浓缩有机相得到粗产物,通过硅胶柱色谱法纯化(洗脱剂:己烷/乙酸乙酯=20/1),得到5-甲基-2-噻吩甲醛(1a-CHO,62.3 mg,无色油状物,收率99%)。
参考文献:
[1] Tetrahedron Letters, 2012, vol. 53, # 9, p. 1173 - 1176
[2] Dyes and Pigments, 2014, vol. 106, p. 154 - 160
[3] Patent: EP1555257, 2005, A1. Location in patent: Page/Page column 25-26
[4] Pharmaceutical Chemistry Journal, 1989, vol. 23, # 7, p. 592 - 596
[5] Khimiko-Farmatsevticheskii Zhurnal, 1989, vol. 23, # 7, p. 840 - 843
