1375108-40-9
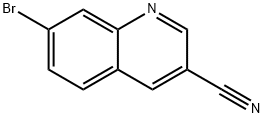 1375108-40-9 结构式
1375108-40-9 结构式
基本信息
7-methylquinoline-3-carbonitrile
3-Quinolinecarbonitrile, 7-bromo-
物理化学性质
制备方法
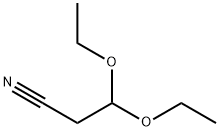
2032-34-0
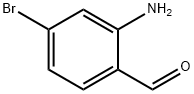
59278-65-8

1375108-40-9
以3,3-二乙氧基丙腈(1.80 mL,12.00 mmol)、2-氨基-4-溴苯甲醛(2 g,10.00 mmol)和对甲苯磺酸一水合物(0.380 g,2.000 mmol)为原料,在甲苯(30 mL)中混合。反应混合物经Dean-Stark装置在回流条件下加热3小时。反应完成后,冷却至室温,减压蒸发除去溶剂。残余物溶于少量DMF中,用氯仿稀释,依次用水和碳酸氢钠溶液洗涤。水层用氯仿萃取,合并有机相,用盐水洗涤,无水硫酸钠干燥,减压浓缩。通过快速柱色谱法(洗脱剂:二氯甲烷/甲醇,0-3%)纯化,所得产物在乙醚中研磨,得到7-溴-3-氰基喹啉(1.75 g,收率75%)。1H NMR(400 MHz,DMSO-d6)δ ppm:7.95(dd,J = 8.72, 1.89 Hz,1H),8.08(d,J = 8.84 Hz,1H),8.38(d,J = 2.02 Hz,1H),9.13(d,J = 1.52 Hz,1H),9.21(d,1H)。
参考文献:
[1] Patent: WO2013/28447, 2013, A1. Location in patent: Page/Page column 119-120
[2] Patent: WO2013/52716, 2013, A1. Location in patent: Page/Page column 50; 65
[3] Patent: WO2013/177253, 2013, A2. Location in patent: Page/Page column 61
[4] Patent: WO2014/8223, 2014, A2. Location in patent: Page/Page column 73
