1446-61-3
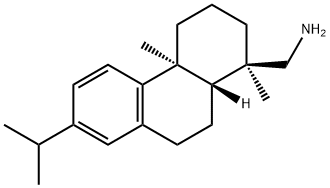 1446-61-3 结构式
1446-61-3 结构式
基本信息
脱氢松香胺
去氢松脂胺
松香胺D
1 4A-DIMETHYL-7-ISOPROPYL-1 2 3,4A 9 10 10A-OCTAHYDRO-1-PHENANTHRENE METHYLAMINE
(1R,4AS,10AR)-1,2,3,4,4A,9,10,10A-OCTAHYDRO-1-,4A-DIMETHYL-7-(1-METHYLETHYL)-1-PHENANTHRENEMETHANAMINE HYDROCHLORIDE
AMINE D
D(+)-DEHYDROABIETYLAMINE
(+)-DEHYDROABIETHYLAMINE
DEHYDROABIETHYLAMINE
(+)-DEHYDROABIETYLAMINE
DEHYDROABIETYLAMINE
LYLAMINE HYDROCHLORIDE
(1r-(1alpha,4abeta,10aalpha))-hylethyl)
[1theta-(1alpha,4abeta,10aalpha)]-hylethyl)
1,2,3,4,4a,9,10,10a-octahydro-1,4a-dimethyl-7-(1-methylethyl)-,[1R-(1.alpha.,4a.beta.,10a.alpha.)]1-Phenanthrenemethanamine
13-isopropylpodocarpa-8,11,13-trien-15-amine
13-trien-15-amine,13-isopropyl-podocarpa-11
1-phenanthrenemethanamine,1,2,3,4,4a,9,10,10a-octahydro-1,4a-dimethyl-7-(1-met
Dehydrobietylamine
DEHYDROABIETYLAMINE, TECH., 60%
DehydroabietylamineTech60%
1-Phenanthrenemethanamine, 1,2,3,4,4a,9,10,10a-octahydro-1,4a-dimethyl-7-(1-methylethyl)-, (1R,4aS,10aR)-
物理化学性质
安全数据
制备方法
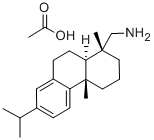
2026-24-6

1446-61-3
以脱氢松香胺醋酸盐为原料合成脱氢松香胺的一般步骤如下:将粗制的60%(+)-脱氢枞胺(42.0g)溶解于甲苯(70.0cm3)中,缓慢加入乙酸(9.65g)的甲苯(30.0cm3)溶液。将混合物置于冰箱中结晶。过滤收集产物,并用己烷(30.0cm3)洗涤。将(+)-脱氢枞胺乙醇合物从甲醇中重结晶。将(+)-脱氢枞胺乙酸盐(21.0g)溶解于热水中,加入10% NaOH水溶液(28.0cm3)。用二乙醚(50.0cm3)萃取(+)-脱氢枞胺,用水洗涤有机相至中性,随后用无水硫酸钠干燥。蒸发溶剂,真空干燥得到的(+)-脱氢枞胺,获得白色固体;产量37.0克,收率88.2%;熔点44.28℃(文献值44-45℃[16])。[α]22D +44.3480(c,10.0mg/cm3,CHCl?)。1H NMR(500MHz,CDCl?)δ 0.89(s,3H,CH?),1.22(s,3H,CH?),1.22(d,J=7.0Hz,6H,2×CH?),1.33(m,2H,CH?),1.39(m,1H,CHH),1.52(dd,J=11.8,3.3Hz,1H,CH),1.69(m,2H,CH?),1.74(m,2H,CH?),2.30(dt,J=13.1,1.7Hz,1H,CHH),2.40(d,J=13.5Hz,1H,CHH),2.61(d,J=13.5Hz,1H,CHH),2.82(sep,J=7.0Hz,CH),2.88(m,2H,CH?),6.89(d,J=1.9Hz,1H,CHAr),7.00(dd,J=8.1,1.9Hz,1H,CHAr),7.18(d,J=8.1Hz,1H,CHAr)。13C NMR(500MHz,CDCl?)δ 18.78(CH?),18.90(CH?),18.90(CH?),24.11(CH?),24.13(CH?),25.37(CH?),30.31(CH?),33.58(CH),35.36(CH?),37.36(C),37.53(C),38.70(CH?),45.00(CH),53.99(CH?),123.96(CHAr),124.38(CHAr),126.94(CHAr),134.84(CAr),145.67(CAr),147.63(CAr)。HRMS-ESI m/z 286.2540;C??H??N [M+H]?计算值286.2529。
参考文献:
[1] Australian Journal of Chemistry, 2017, vol. 70, # 7, p. 845 - 856
