150529-73-0
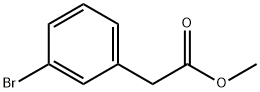 150529-73-0 结构式
150529-73-0 结构式
基本信息
3-溴苯乙酸甲酯
(3-溴苯基)乙酸甲酯
2-(3-溴苯基)乙酸甲酯
2-(3-溴苯基)乙酸甲酯METHYL 3-BROMOPHENYLACETATE
2-(3-溴苯基)乙酸甲酯 Methyl 3-bromophenylacetate
Methyl3-bromophenylacetate98%
Methyl 3-bromophenylacetate 98%
METHYL 2-(3-BROMOPHENYL)ACETATE
methyl 2-(3-bromophenyl)acetatee
(3-BROMOPHENYL)ACETIC ACID METHYL ESTER
Benzeneacetic acid, 3-bromo-, methyl ester
3-Bromophenylacetic acid methyl ester, Methyl 2-(3-bromophenyl)ethanoate
物理化学性质
制备方法

67-56-1
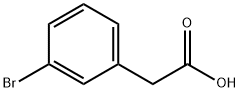
1878-67-7

150529-73-0
以甲醇和3-溴苯乙酸为原料合成(3-溴苯基)乙酸甲酯的一般步骤:将乙酰氯(0.08 mL,1.2 mmol,0.5当量)和3-溴苯乙酸(0.5 g,2.3 mmol,1.0当量)加入甲醇(10 mL)中,将反应混合物回流2小时。反应完成后,通过TLC监测确认起始原料消耗完毕。将反应混合物浓缩,用二氯甲烷(DCM)萃取。合并有机相,用饱和食盐水洗涤,无水硫酸钠干燥,过滤后减压浓缩,得到(3-溴苯基)乙酸甲酯,为无色油状物(0.525 g,收率98.7%)。产物经LC-MS(ES)分析,m/z = 229.0, 231.0 [M + H]+。1H NMR(400 MHz, DMSO-d6)δ 3.61(s, 3H), 3.70(s, 2H), 7.26-7.30(m, 2H), 7.43-7.46(m, 1H), 7.48(s, 1H)。
参考文献:
[1] Bioorganic and Medicinal Chemistry, 2003, vol. 11, # 13, p. 2843 - 2866
[2] Journal of Medicinal Chemistry, 2008, vol. 51, # 3, p. 392 - 395
[3] Patent: EP1479681, 2004, A1. Location in patent: Page 111; 112
[4] Patent: US2007/3539, 2007, A1. Location in patent: Page/Page column 96
[5] Journal of Medicinal Chemistry, 1995, vol. 38, # 26, p. 4976 - 4984
