152120-61-1
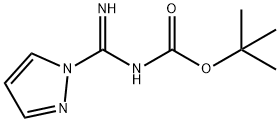 152120-61-1 结构式
152120-61-1 结构式
基本信息
N-BOC-吡唑-1-甲脒
N-BOC-1-鸟苷基吡唑
N-BOC-1H-吡唑-1-甲脒
N-(叔丁氧羰基)-1H-吡唑-1-甲脒
N-BOC-1H-吡唑-1-甲脒, 98+%
N-(叔丁氧基羰基)-1H-吡唑-1-羧酰胺
单BOC脒 N-BOC-1H-吡唑-1-甲脒
B1产品(N-BOC-1H-吡唑-1-甲脒)
N-[氨基-(1-吡唑)亚甲基]氨基甲酸叔丁酯
1-(N-Boc-carb
e-1-carboxamidine
N-Boc-1-guanylpyrazole
1-(N-Boc-amidino)pyrazole
N-Boc-pyrazole-1-carboxaMidine
H-pyrazole-1-carboxamidine HCl
Boc-1H-pyrazole-1-carboxamidine
N-BOC-1H-PYRAZOLE-1-CARBOXAMIDINE
N-Boc-1H-pyrazole-1-carboximidamide
物理化学性质
制备方法

24424-99-5
4023-02-3

152120-61-1
一般步骤:在室温下,将1H-吡唑-1-甲脒盐酸盐(9.0g,61.5mmol,1.0当量)和9.0mL水加入至二碳酸二叔丁酯(13.4g,61.5mmol)的丙酮(45mL)溶液中。随后,缓慢滴加K2CO3(4.24g,30.6mmol,0.5当量)的水(12.0mL)溶液,滴加时间为30分钟。反应2小时后,补充加入二碳酸二叔丁酯(1.35g,6mmol,0.1当量),并将反应混合物搅拌过夜。反应完成后,通过减压蒸馏除去丙酮。将所得白色固体溶解于30.0mL水中,并在0℃下搅拌30分钟。过滤收集沉淀的叔丁基(亚氨基(1H-吡唑-1-基)甲基)氨基甲酸酯,依次用水和己烷洗涤,最后真空干燥,得到无色固体N-Boc-1H-吡唑-1-甲脒(11.37g,收率88%)。产物经1H NMR(CDCl3, 400MHz)表征:δ9.06(brs, 1H),8.45(d, J = 3.2Hz, 1H),7.67(d, J = 0.8Hz, 1H),7.59(brs, 1H),6.39(dd, J = 2.8, 1.6Hz, 1H),1.54(s, 9H)。
参考文献:
[1] Tetrahedron Letters, 2007, vol. 48, # 13, p. 2357 - 2359
[2] Organic and Biomolecular Chemistry, 2013, vol. 11, # 24, p. 3943 - 3948
[3] Patent: WO2015/147950, 2015, A2. Location in patent: Page/Page column 38
[4] Patent: US2013/79324, 2013, A1. Location in patent: Paragraph 0827; 0828
[5] Tetrahedron Letters, 1993, vol. 34, # 21, p. 3389 - 3392
