15520-05-5
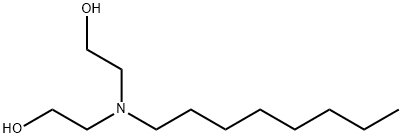 15520-05-5 结构式
15520-05-5 结构式
基本信息
2,2'-(辛基亚氨基)双乙醇
2,2' - (辛基氮杂二基)二乙醇
2,2'-(octylimino)bisethanol
3-Octyl-3-azapentane-1,5-diol
2,2'-(Octylazanediyl)diethanol
Ethanol, 2,2'-(octylimino)bis-
bis-(2-hydroxy-ethyl)-octyl-amine
2,2-(OCTYLAZANEDIYL)BIS(ETHAN-1-OL)
2-[2-hydroxyethyl(octyl)amino]ethanol
N,N Bis (2 hydroxyethyl) alk (C8) amine
物理化学性质
SURFACTANT - CLEANSING
CLEANSING
SURFACTANT - EMULSIFYING
制备方法

111-83-1
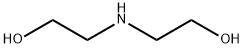
111-42-2

15520-05-5
以1-溴辛烷和二乙醇胺为原料合成2,2'-(辛基氮杂二基)二乙醇的一般步骤如下:将1-溴辛烷(3.0 g,15.0 mmol)、二乙醇胺(2.4 g,22.8 mmol)和无水碳酸钾(4.28 g,31.0 mmol)溶于乙腈(40 mL)中,在氮气保护下回流反应12小时。反应完成后,冷却至室温,将反应混合物浓缩。用二氯甲烷萃取有机相,并用无水硫酸钠干燥。减压过滤除去硫酸钠后,得到粗产物。粗产物通过柱色谱法纯化,使用二氯甲烷和甲醇作为洗脱剂,得到纯的2,2'-(辛基氮杂二基)二乙醇,为无色油状物(590 mg,收率91%)。1H NMR (CDCl3)数据如下:δ 4.22 (brs, 2H, -OHCH2), 3.86 (t, 4H, J = 5 Hz, -CH2OH), 3.10 (t, 4H, J = 4.5 Hz, -CH2N), 2.94 (t, 2H, J = 8 Hz, -CH2N), 1.69-1.60 (m, 2H, -CH2), 1.30-1.25 (m, 10H, -CH2), 0.87 (t, 3H, J = 7 Hz, -CH3)。
参考文献:
[1] Patent: WO2018/42367, 2018, A2. Location in patent: Page/Page column 95; 96-97
[2] Molecular Crystals and Liquid Crystals (1969-1991), 1990, vol. 185, p. 131 - 140
[3] Patent: CN106187790, 2016, A. Location in patent: Paragraph 0044-0050
[4] Patent: US2541088, 1946,
[5] European Journal of Medicinal Chemistry, 1976, vol. 11, p. 115 - 124
