166815-96-9
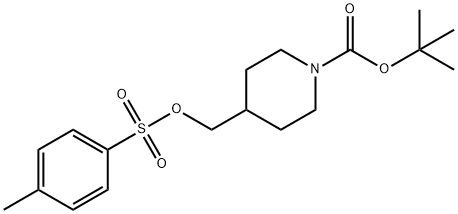 166815-96-9 结构式
166815-96-9 结构式
基本信息
1-BOC-4-对甲苯磺酰氧基哌啶
1-BOC-4-(对二甲苯磺酸基)哌啶
1-BOC-4-(P-甲苯磺酰氧甲基)哌啶
N-BOC-4-(4-甲基苯磺酸甲基)哌啶
N-BOC-4-(4-甲苯磺酰氧基甲基)哌啶
N-BOC-4-(4-甲基苯磺酰氧甲基)哌啶
1-BOC-4-[(对甲苯磺酰基氧基)甲基]哌啶
1-BOC-4-(O-对甲苯磺酰基-羟甲基)哌啶
1-N-BOC-4-(4-甲基苯磺酰氧甲基)哌啶
tert-Butyl 4-((tosyloxy)
1-Boc-4-(tosyloxyMethyl)piperidine
N-Boc-4-(4-toluenesulfonyloxymethyl)piperidine
1-N-bOC-4-(4-TOLUENESULFONYLOXYMETHYL)PIPERIDINE
1-Boc-4-(p-toluenesulfonyloxymethyl)piperidine,96%
1-Boc-4-(p-toluenesulfonyloxyMethyl)piperidine, 96%
N-tert-Butoxycarbonyl-4-(4-toluenesulfonyloxymethyl)
N-(tert-Butoxycarbonyl)-4-(tosyloxymethyl)piperidine
1-(tert-Butoxycarbonyl)-4-(tosyloxymethyl)piperidine
物理化学性质
制备方法

98-59-9
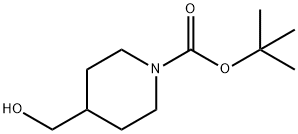
123855-51-6

166815-96-9
实施例1A 4-((甲苯磺酰氧基)甲基)哌啶-1-羧酸叔丁酯的合成:在氮气保护下,将N-BOC-4-哌啶甲醇(10g,46.5mmol)和三乙胺(5.64g,55.8mmol)溶解于二氯甲烷(100mL)中,冷却至0℃。随后,向该溶液中缓慢加入对甲苯磺酰氯(9.73g,51.2mmol)和4-二甲氨基吡啶(1.13g,9.3mmol)。反应混合物在17℃下搅拌2小时。反应完成后,加入水(100mL)淬灭反应。用二氯甲烷(100mL×2)萃取水层。合并有机层,用无水硫酸钠干燥,过滤后减压浓缩,得到粗产物。通过柱色谱纯化,得到1-N-Boc-4-(4-甲基苯磺酰氧甲基)哌啶(17g,99%收率),为白色固体。LCMS(ESI)m/z:370(M + 1)。
参考文献:
[1] Patent: US2017/29430, 2017, A1. Location in patent: Paragraph 0255
[2] Patent: CN105315267, 2016, A. Location in patent: Paragraph 0033; 0049
[3] Patent: WO2008/42282, 2008, A2. Location in patent: Page/Page column 107-108; 168-169; 210-211
[4] Patent: WO2012/168349, 2012, A1. Location in patent: Page/Page column 37
[5] Patent: US2013/34504, 2013, A1. Location in patent: Paragraph 0147; 0148; 0149
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | B4759 | 1-叔丁氧羰基-4-[(对二甲苯磺酸基)甲基]哌啶 1-(tert-Butoxycarbonyl)-4-[(p-toluenesulfonyloxy)methyl]piperidine | 166815-96-9 | 5g | 240元 |
| 2025/12/22 | XW0216681596905 | 1-BOC-4-(P-甲苯磺酰氧甲基)哌啶 | 166815-96-9 | 100G | 697元 |
| 2025/12/22 | XW0216681596904 | 1-BOC-4-(P-甲苯磺酰氧甲基)哌啶 | 166815-96-9 | 25G | 239元 |
