170123-25-8
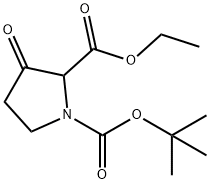 170123-25-8 结构式
170123-25-8 结构式
基本信息
N-BOC-3-氧代吡咯烷-2-甲酸乙酯
1-N-BOC-3-氧代-吡咯烷-2-甲酸乙酯
1-叔丁基2-乙基3-氧吡咯烷-2-1,2-二羧酸酯
Ethyl N-Boc-3-oxopyrrolidine-2-carboxylate
1-tert-Butyl 2-ethyl 3-oxopyrrolidine-1,2-dicarboxylate
Ethyl 1-(tert-butoxycarbonyl)pyrrolidin-3-one-2-carboxylate
1-(tert-Butoxycarbonyl)pyrrolidin-3-one-2-carboxylic acid ethyl ester
1,2-Pyrrolidinedicarboxylic acid, 3-oxo-, 1-(1,1-dimethylethyl) 2-ethyl ester
物理化学性质
制备方法
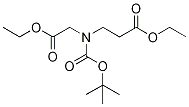
146256-97-5

170123-25-8
在10℃下,向叔丁醇钾(791 mg,7.05 mmol)的无水甲苯(19 mL)悬浮液中缓慢滴加3-(叔丁氧基羰基-乙氧基羰基甲基-氨基)-丙酸乙酯(1.43 g,4.7 mmol)的甲苯溶液。反应混合物在0℃下搅拌90分钟,随后用乙酸(500 mL)淬灭反应。接着,加入预先冷却的NaH2PO4·H2O(2.5 g)水溶液(25 mL)。分离有机层和水层,水相用氯仿(CHCl3)萃取。合并所有有机层,用无水Na2SO4干燥,减压浓缩。粗产物通过快速柱色谱法(硅胶,石油醚/乙酸乙酯 5:1,Rf 0.44)纯化,得到N-Boc-3-氧代吡咯烷-2-甲酸乙酯,为黄色油状物(497 mg,收率41%)。产物经1H NMR(CDCl3, 200 MHz)确认,主旋转异构体信号如下:δ 4.55和4.47(s, 1H),4.27-4.22(m, 2H),3.91-3.84(m)和3.82(t, J = 8.2 Hz, 2H),2.69(t, J = 8.0 Hz, 2H),1.49和1.43(s, 9H),1.30(t, J = 7.1 Hz, 3H)。元素分析结果符合C12H19NO5的计算值(C, H, N)。
参考文献:
[1] Chemical Communications, 2016, vol. 52, # 53, p. 8271 - 8274
[2] Journal of the Chemical Society - Perkin Transactions 1, 1997, # 15, p. 2179 - 2187
[3] Organic Letters, 2007, vol. 9, # 21, p. 4255 - 4258
[4] Tetrahedron Asymmetry, 2005, vol. 16, # 13, p. 2243 - 2247
[5] Bioorganic and Medicinal Chemistry Letters, 2008, vol. 18, # 6, p. 1931 - 1938
