172015-79-1
中文名称
(1S–4R)-4-(2-氨基-6-氯-9H-嘌呤-9-基)-2-环戊烯-1-甲醇盐酸盐
英文名称
(1S–4R)-4-(2-amino-6-chloro-9H-purin-9-yl)-2-cyclopentene-1-methanol hydrochloride
CAS
172015-79-1
分子式
C11H12ClN5O.ClH
分子量
302.164
MOL 文件
172015-79-1.mol
更新日期
2026/02/26 11:17:21
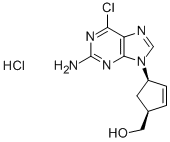 172015-79-1 结构式
172015-79-1 结构式
基本信息
中文别名
阿巴卡韦杂质C阿巴卡韦相关物质C USP标准品
(1S,4R)-4-(2-氨基-6-氯-9H-嘌呤-9-基)-2-环戊烯]甲醇盐酸盐
(1S–4R)-4-(2-氨基-6-氯-9H-嘌呤-9-基)-2-环戊烯-1-甲醇盐酸盐
英文别名
Abacavir Related CoMpound C( Abacavir USP Related Compound C)
Abacavir Impurity 8( Abacavir USP Related Compound C)
(1S,4R)-4-(2-AMino-6-chloro-9H-purin-yl)-2-cyclopentene-1-Methanol,HCl
[(1R,4S)-4-(2-Amino-6-chloro-9H-purin-9-yl)cyclopent-2-en-1-yl]methanol
(1S,4R)-4-(2-Amino-6-chloro-9H-purin-9-yl)-2-cyclopentene-1-methanol HCl
(1S,4R)-4-(2-amino-6-chloropurin-9-yl)cyclopent-2-en-1-yl]methanol,hydrochloride
(1S–4R)-4-(2-amino-6-chloro-9H-purin-9-yl)-2-cyclopentene-1-methanol hydrochloride
[(1S,4R)-4-(2-Amino-6-chloro-9H-purin-9-yl)cyclopent-2-enyl]methanol hydrochloride
(1S,4R)-4-(2-amino-6-chloro-9H-purin-9-yl) -2-Cyclopentene-1- methano Hydrochloride
所属类别
分析化学:药典标准品和杂质标准品物理化学性质
熔点165-170?C
储存条件Inert atmosphere,2-8°C
溶解度可溶于DMSO(少许)、甲醇(少许)
形态neat
颜色灰白色至米色至棕色
主要应用pharmaceutical (small molecule)
InChI1S/C11H12ClN5O.ClH/c12-9-8-10(16-11(13)15-9)17(5-14-8)7-2-1-6(3-7)4-18;/h1-2,5-7,18H,3-4H2,(H2,13,15,16);1H/t6-,7+;/m1./s1
InChIKeyVZJFPECAPXUELG-HHQFNNIRSA-N
SMILESClc1nc(nc2[n](cnc21)[C@@H]3C[C@@H](C=C3)CO)N.Cl
制备方法
方法1
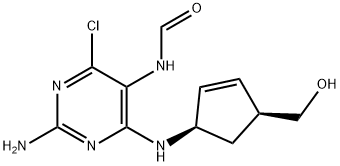
171887-04-0

172015-79-1
以阿巴卡韦中间体A4为原料,合成((1S,4R)-4-(2-氨基-6-氯-9H-嘌呤-9-基)环戊-2-烯-1-基)甲醇盐酸盐的一般步骤如下:向反应釜中加入N-[2-氨基-4-氯-6-[[(1R,4S)-4-(羟甲基)-2-环戊烯-1-基]氨基]-5-嘧啶基]甲酰胺(0.1762 mol)和三氯甲基硅酸酯(7体积)。将反应混合物冷却至0-5℃,在0-10℃下缓慢加入盐酸(0.7体积)。加毕,于0-5℃下保温反应60分钟。随后,将反应混合物升至室温并继续搅拌16-18小时。反应完成后,过滤收集固体产物,并在甲醇中进行重结晶纯化。(产率:76%,HPLC纯度:98.69%)。
参考文献:
[1] Nucleosides, Nucleotides and Nucleic Acids, 2000, vol. 19, # 1-2, p. 297 - 327
[2] Patent: US2017/233329, 2017, A1. Location in patent: Paragraph 0054; 0055
[3] Patent: CN107641122, 2018, A. Location in patent: Paragraph 0016; 0049; 0055-0056; 0059-0073; 0076


