178876-82-9
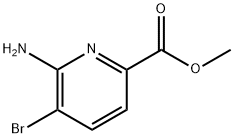 178876-82-9 结构式
178876-82-9 结构式
基本信息
6-氨基-5-溴-2-吡啶羧酸甲酯
5-溴-6-氨基吡啶-2-羧酸甲酯
6-氨基-5-溴吡啶-2-甲酸甲酯
6-氨基-5-溴吡啶-2-羧酸甲酯
5-溴-6-氨基吡啶-2-甲酸甲酯
METHYL 6-AMINO-5-BROMOPYRIDINE-2-CARBOXYLATE
METHYL 6-AMINO-5-BROMOPICOLINATE ISO 9001:2015 REACH
6-AMino-5-broMopyridine-2-carboxylic Acid Methyl Ester
2-Pyridinecarboxylic acid, 6-amino-5-bromo-, methyl ester
物理化学性质
制备方法
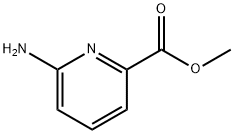
36052-26-3
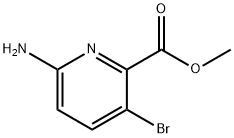
178876-83-0

178876-82-9
d)6-氨基-5-溴吡啶-2-甲酸甲酯的合成:在室温下,将6-氨基吡啶-2-甲酸甲酯(10g,66.0mmol)溶于氯仿(450mL)中,缓慢加入溴(3.4mL,66.0mmol)的氯仿(100mL)溶液。反应混合物搅拌40小时后,用氯仿稀释,依次用饱和硫代硫酸钠溶液和水洗涤。有机相经无水硫酸钠干燥,减压浓缩,残余物通过硅胶柱色谱纯化,以乙酸乙酯/己烷为洗脱剂,得到5-溴-6-氨基吡啶-2-羧酸甲酯,为黄色固体(3.3g,收率22%)。MS ESI(m/e):231.0 [(M + H)+]。1H NMR(CDCl3,400MHz):δ(ppm)= 7.76(d,J = 7.88Hz,1H),7.34(d,J = 7.92Hz,1H),5.23(s,2H),3.94(s,3H)。 d)6-氨基-3-溴吡啶-2-甲酸甲酯的分离:在步骤d)中,作为副产物分离得到异构体6-氨基-3-溴吡啶-2-甲酸甲酯(3.0g,收率19%)。MS ESI(m/e):231.2 [(M + H)+]。1H NMR(CDCl3,400MHz):δ(ppm)= 7.60(d,J = 8.72Hz,1H),6.47(d,J = 7.88Hz,1H),4.71(s,2H),3.94(s,3H)。
参考文献:
[1] Journal of Organic Chemistry, 1996, vol. 61, # 14, p. 4623 - 4633
[2] Organic Process Research and Development, 2010, vol. 14, # 1, p. 263 - 271
[3] Tetrahedron Letters, 2010, vol. 51, # 38, p. 5035 - 5037
[4] Patent: US2011/190269, 2011, A1. Location in patent: Page/Page column 61-62
[5] Patent: WO2011/92272, 2011, A1. Location in patent: Page/Page column 129; 130