185116-43-2
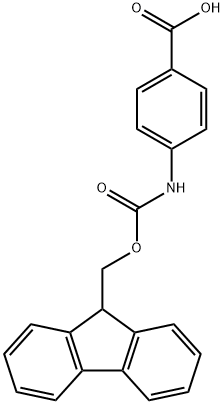 185116-43-2 结构式
185116-43-2 结构式
基本信息
4-(FMOC-AMINO)BENZOIC ACID
FMOC-4-ABZ-OH
FMOC-4-AMINOBENZOIC ACID
FMOC-PABA-OH
FMOC-P-ABZ-OH
N-ALPHA-9-FLUORENYLMETHOXYCARBONYL-4-AMINOBENZOIC ACID
N-ALPHA-9-FLUORENYLMETHOXYCARBONYL-P-AMINOBENZOIC ACID
N-ALPHA-FMOC-P-AMINOBENZOIC ACID
N-FMOC-4-AMINOBENZOIC ACID
RARECHEM EM WB 0013
Benzoic acid, 4-[[(9H-fluoren-9-ylmethoxy)carbonyl]amino]- (9CI)
4-(FMOC-AMINO)BENZOIC ACID 96+%
物理化学性质
安全数据
制备方法
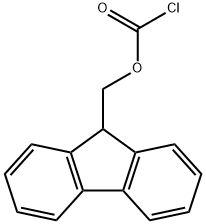
28920-43-6
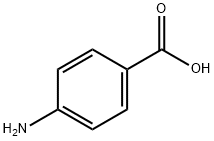
150-13-0

185116-43-2
在惰性气氛下,将对氨基苯甲酸(10 g,73 mmol)溶解于无水N-甲基吡咯烷酮(NMP,50 mL)中。随后,缓慢滴加氯甲酸-9-芴基甲酯(Fmoc-Cl,18.9 g,73 mmol)的无水NMP(50 mL)溶液。反应混合物在室温下搅拌24小时。反应完成后,将混合物缓慢倒入400 mL水中,以沉淀产物。通过过滤收集无色沉淀,并用大量水洗涤。最后,将产物在120℃下真空干燥,得到N-Fmoc-对氨基苯甲酸(24.8 g,产率94%)。产物的熔点为215℃(分解)。核磁共振氢谱(1H-NMR,300 MHz,DMSO-d6):δ 4.33(t,J = 6.25 Hz,1H),4.54(d,J = 6.25 Hz,2H),7.33-7.45(m,4H),7.57(d,J = 7.35 Hz,2H),7.76(d,J = 7.35 Hz,2H),7.89(m,4H),10.08(s,1H),12.69(s,1H)。核磁共振碳谱(13C-NMR和DEPT,300 MHz,DMSO-d6):δ 46.61(CH2),65.83(CH),117.5(CH),120.22(CH),124.48(C),125.14(CH),127.17(CH),127.74(CH),130.48(CH),140.86(C),143.31(C),143.74(C),153.31(C),167.03(C)。红外光谱(IR,ν,cm-1):3344,2970,2887,2660,2544,1709,1673,1610,1592,1526,1511,1411,1311,1282,1221,1052,850,736。反相高效液相色谱(RP-HPLC)保留时间:26.6分钟。质谱(FD-MS):m/z(%)= 359.1(100),360.1(17.4);计算值[C22H17NO4] = 359.1。
参考文献:
[1] Tetrahedron Letters, 2013, vol. 54, # 8, p. 753 - 756
[2] Chemical Communications, 2013, vol. 49, # 40, p. 4555 - 4557
[3] Journal of Chemical Research, Miniprint, 1998, # 10, p. 2736 - 2753
[4] Chemical Communications, 2014, vol. 50, # 14, p. 1691 - 1693
