19228-91-2
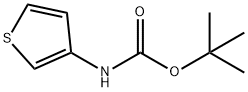 19228-91-2 结构式
19228-91-2 结构式
基本信息
N-BOC-3-氨基噻吩
N-(叔丁氧羰基)-3-氨基噻吩
叔丁基N-(3 - 噻吩基)氨基甲酸
N-Boc-3-aminothiophene 96%
TERT-BUTYL 3-THIENYLCARBAMATE
TERT-BUTYL N-(3-THIENYL)CARBAMATE
tert-butyl thiophen-3-ylcarbaMate
3-Aminothiophene, N-BOC protected
Tert-butyl N-thiophen-3-ylcarbamate
3-(tert-Butoxycarbonylamino)thiophene
3-Thiophenecarbamic acid tert-butyl ester
Thiophen-3-yl-carbamic acid tert-butyl ester
物理化学性质
安全数据
制备方法
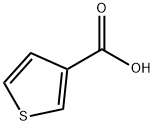
88-13-1

75-65-0

19228-91-2
制备实施例U-1:叔丁基N-(3-噻吩基)氨基甲酸酯的合成。将3-噻吩甲酸(2.50 g,19.5 mmol)、二苯基磷酰基叠氮化物(4.62 mL,21.5 mmol)和三乙胺(3.26 mL,23.4 mmol)溶解于叔丁醇(50 mL)中。将反应混合物在回流条件下搅拌3.5小时。反应完成后,将反应液冷却至0℃,加入水淬灭反应。随后用乙酸乙酯萃取反应混合物,合并有机层并用饱和食盐水洗涤。有机层经无水硫酸镁干燥后,减压浓缩除去溶剂。残余物通过硅胶柱色谱法(洗脱剂:石油醚/乙酸乙酯=10:1)纯化,得到叔丁基N-(3-噻吩基)氨基甲酸酯(3.33 g,16.7 mmol,收率86%)为白色固体。1H-NMR(DMSO-d6)δ(ppm):1.46(9H,s),6.97(1H,d,J=5.2 Hz),7.16(1H,s),7.38(1H,m),9.61(1H,s)。
参考文献:
[1] Patent: EP1782811, 2007, A1. Location in patent: Page/Page column 66
[2] Patent: EP1669348, 2006, A1. Location in patent: Page/Page column 74
[3] Journal of Materials Chemistry C, 2017, vol. 5, # 10, p. 2509 - 2512
[4] Patent: US2006/154875, 2006, A1. Location in patent: Page/Page column 7; 11; 12
[5] Bioorganic and Medicinal Chemistry Letters, 2006, vol. 16, # 21, p. 5567 - 5571
