210366-15-7
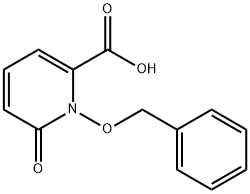 210366-15-7 结构式
210366-15-7 结构式
基本信息
1-(benzyloxy)-2-oxo-1,2-dihydropyridine-6-carboxylic acid
1-(Benzyloxy)-6-oxo-1,6-dihydropyridine-2-carboxylic acid
1,6-dihydro-6-oxo-1-(phenylMethoxy)-2-Pyridinecarboxylic acid
2-Pyridinecarboxylic acid, 1,6-dihydro-6-oxo-1-(phenylMethoxy)-
物理化学性质
制备方法

100-44-7
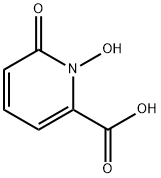
94781-89-2

210366-15-7
以氯化苄和1-羟基-6-氧代-1,6-二氢吡啶-2-羧酸为原料合成1-(苄氧基)-6-氧代-1,6-二氢吡啶-2-羧酸的一般步骤:将1-羟基-6-羧基-2(1H)-吡啶酮(15.5 g,0.1 mol)和无水碳酸钾(27.6 g,0.2 mol)与苄基氯(15.2 g,0.12 mol)在甲醇(250 mL)中混合,回流反应16小时。反应完成后,过滤混合物,将滤液蒸发至干。将残余物溶于水(50 mL)中,用6N HCl酸化至pH 2。过滤分离出白色沉淀,用冷水洗涤,真空干燥,得到22.3 g(产率91%)1-苄氧基-6-羧基-2(1H)-吡啶酮,熔点为176-177℃。产物经1H NMR(300 MHz, CDCl3)和13C NMR(75 MHz, DMSO-d6)表征,数据如下:1H NMR δ 5.269 (s, 2H), 6.546 (dd, J = 16 Hz, J = 6.7 Hz, 1H), 6.726 (dd, J = 16 Hz, J = 9.2 Hz, 1H), 7.39-7.51 (m, 6H); 13C NMR δ 77.9, 106.0, 124.1, 128.5, 129.1, 129.6, 133.8, 138.7, 140.5, 157.7, 161.7。元素分析(C13H11NO4)计算值:C, 63.66 (63.75); H, 4.53 (4.55); N, 5.71 (5.52)。
参考文献:
[1] Journal of Medicinal Chemistry, 2014, vol. 57, # 11, p. 4849 - 4860
[2] Journal of Medicinal Chemistry, 2002, vol. 45, # 18, p. 3963 - 3971
[3] Patent: WO2008/8797, 2008, A2. Location in patent: Page/Page column 58
[4] Patent: WO2007/121453, 2007, A2. Location in patent: Page/Page column 108
[5] Patent: WO2017/105565, 2017, A2. Location in patent: Paragraph 0109