214834-18-1
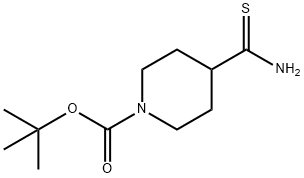 214834-18-1 结构式
214834-18-1 结构式
基本信息
BOC-哌啶-4-硫代甲酰胺
1-BOC-哌啶-4-硫代酰胺
N-BOC-4-哌啶硫代甲酰胺
1-BOC-4-硫代甲酰胺哌啶
N-BOC-哌啶-4-硫代乙酰胺
1-BOC-哌啶-4-硫代甲酰胺
N-Boc-哌啶-4-硫代甲酰胺
1-叔丁氧羰基哌啶-4-硫代甲酰胺
4-氨基甲酰硫基哌啶-1-羧酸叔丁酯
1-Boc-4-thiocarbamoylpiperidine
N-Boc-4-PiperidinecarbothioaMide
N-BOC-piperidine-4-carbothioamide
1-Boc-piperidine-4-carbothioamide
N-BOC-Piperidine-4-thiocarboxamide
N1-BOC-PIPERIDINE-4-THIOCARBOXAMIDE
PIPERIDINE-4-THIOCARBOXAMIDE, 1-BOC PROTECTED
Piperidine-4-thiocarboxamide, N1-BOC protected
4-Carbamothioylpiperidine, N1-BOC protected 97%
物理化学性质
制备方法
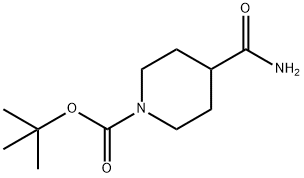
91419-48-6

214834-18-1
以4-氨基甲酰基哌啶-1-羧酸叔丁酯为原料合成4-氨基硫代羰基四氢吡啶-1(2H)-甲酸叔丁酯的一般步骤:将叔丁基-4-氨基甲酰基哌啶-1-羧酸酯(2.65g,11.62mmol)溶解于DME/DCM(2:1,78mL)的混合溶剂中,随后加入Lawesson试剂(2.35g,5.81mmol,0.5当量)。将反应混合物在室温下搅拌过夜。反应完成后,减压除去溶剂,将残余物溶解于乙酸乙酯中,并用饱和K2CO3水溶液洗涤。分离有机层,用无水Na2SO4干燥,过滤后浓缩至干。将得到的残余物用乙醚研磨,干燥后得到4-氨基硫代羰基四氢吡啶-1(2H)-甲酸叔丁酯(2.65g,收率92%)为白色固体。产物经HPLC分析显示保留时间(Rt)为5.06分钟。1H NMR(401MHz,DMSO-d6)δppm:9.39(br.s.,1H),9.09(br.s.,1H),4.00(d,J=12.6Hz,2H),2.77-2.61(m,3H),1.71-1.51(m,4H),1.39(br.s.,9H)。
参考文献:
[1] Patent: WO2012/113774, 2012, A1. Location in patent: Page/Page column 40
[2] Patent: US2013/324551, 2013, A1. Location in patent: Paragraph 0311; 0312; 0313
[3] Patent: WO2011/153435, 2011, A1. Location in patent: Page/Page column 13-14
[4] Patent: US2011/318418, 2011, A1. Location in patent: Page/Page column 12
[5] Patent: WO2016/30443, 2016, A1. Location in patent: Page/Page column 102
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/05/22 | XW2148341811 | 4-氨基硫代羰基四氢吡啶-1(2H)-甲酸叔丁酯 | 214834-18-1 | 1G | 44元 |
| 2024/04/30 | Y15408 | 4-氨基甲酰硫基哌啶-1-羧酸叔丁酯 tert-Butyl 4-carbamothioylpiperidine-1-carboxylate | 214834-18-1 | 1g | 206元 |
| 2024/04/30 | Y15408 | 4-氨基甲酰硫基哌啶-1-羧酸叔丁酯 tert-Butyl 4-carbamothioylpiperidine-1-carboxylate | 214834-18-1 | 5g | 503元 |
