216755-57-6
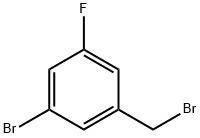 216755-57-6 结构式
216755-57-6 结构式
基本信息
3-溴-5-氟苄溴
3-氟-5-溴溴苄
3-溴-5-氟苄基溴
3-Fluoro-5-broMobenl broMide
5-bromo-3-fluorobenzyl bromide
3-Fluoro-5-bromobenzyl bromide
3-Bromo-5-fluorobenzylbromide,95%
3-Fluoride-5-Bromine Bromine Benzyl
1-Bromo-3-(bromomethyl)-5-fluorobenzene
Benzene, 1-bromo-3-(bromomethyl)-5-fluoro-
1-Bromo-3-(bromomethyl)-5-fluorobenzene, alpha,3-Dibromo-5-fluorotoluene
物理化学性质
制备方法
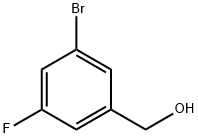
216755-56-5

216755-57-6
以(3-溴-5-氟苯基)甲醇为原料合成3-氟-5-溴溴苄的一般步骤:在二氯甲烷(45mL)中溶解3-溴-5-氟苄醇(1.79g,8.39mmol)和三苯基膦(3.65g,10.10mmol),随后加入四溴化碳(3.34g,10.10mmol)。反应混合物在室温下搅拌过夜。反应完成后,通过真空蒸发去除溶剂。残余物用二乙醚(50mL)处理,并在室温下搅拌形成白色浆液。浆液通过CeliteTM过滤,残余物用二乙醚(2×50mL)洗涤。合并滤液和洗涤液,真空蒸发得到粗产物。粗产物通过硅胶柱层析纯化,使用2%乙酸乙酯/己烷作为洗脱剂,得到3-溴-5-氟苄基溴(2.21g,98%)。产物结构通过1H NMR、19F NMR和13C NMR确认。1H NMR(400MHz,CDCl3):δ7.33(s,1H),7.18(ddd,JHF 8.2Hz,JHH = 2.0,2.0Hz,1H),7.05(ddd,JHF = 9.0Hz,JHH = 1.8,1.6Hz,1H),4.38(s,2H);19F NMR(377MHz,CDCl3):δ-110.19至-110.14(m,1F);13C NMR(101MHz,CDCl3):δ162.67(d,JCF = 252.1Hz),141.61(d,JCF = 8.5Hz),128.17(d,JCF = 3.1Hz),122.94(d,JCF = 10.0Hz),119.39(d,JCF = 24.6Hz),115.34(d,JCF = 22.3Hz),31.31(d,JCF = 2.3Hz)。
参考文献:
[1] Patent: WO2016/54728, 2016, A1. Location in patent: Paragraph 00128
[2] Patent: WO2016/74068, 2016, A1. Location in patent: Paragraph 00122
[3] Patent: CN104447730, 2017, B. Location in patent: Paragraph 0149-0150
[4] Patent: US2007/249607, 2007, A1. Location in patent: Page/Page column 81
[5] Patent: US2002/49204, 2002, A1
