228710-82-5
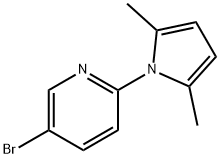 228710-82-5 结构式
228710-82-5 结构式
基本信息
5-溴-2-(2,5-二甲基吡咯-1-基)吡啶
5-溴-2-(2,5-二甲基-1H-吡咯-1-基)吡啶
5-bromo-2-(2,5-dimethyl-1H-pyrrol-1-yl)pyridine
Pyridine, 5-bromo-2-(2,5-dimethyl-1H-pyrrol-1-yl)-
制备方法
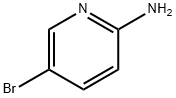
1072-97-5
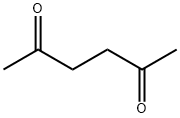
110-13-4

228710-82-5
以2-氨基-5-溴吡啶(13.8 g,0.08 mol)和2,5-己二酮(14.1 mL,0.12 mol)为原料,在甲苯(180 mL)中溶解,加入对甲苯磺酸(100 mg)作为催化剂。将反应混合物置于Dean-Stark装置下回流14小时。反应完成后,冷却至室温,将棕色反应液倒入水(200 mL)中,用甲苯(2×200 mL)萃取。合并有机相,用盐水(50 mL)洗涤,无水硫酸镁干燥,过滤后减压浓缩,得到粗产物。粗产物通过硅胶柱色谱纯化,洗脱剂为乙酸乙酯:戊烷(1:3),得到5-溴-2-(2,5-二甲基-1H-吡咯-1-基)吡啶,为棕色油状物(18.4 g,0.073 mol,收率92%)。产物结构经1H NMR(CDCl3, 400 MHz)确认:δ 2.18(s, 6H),5.90(s, 2H),7.11(d, 1H),7.92(d, 1H),8.62(s, 1H)。低分辨质谱(LRMS)显示m/z 253([M-H]+, 含Br同位素)。
参考文献:
[1] Organic Process Research and Development, 2004, vol. 8, # 4, p. 587 - 592
[2] Patent: WO2004/52372, 2004, A1. Location in patent: Page 85-86
[3] Patent: WO2008/87512, 2008, A1. Location in patent: Page/Page column 51-52
[4] Asian Journal of Chemistry, 2016, vol. 28, # 6, p. 1403 - 1404
[5] Patent: WO2006/82511, 2006, A1. Location in patent: Page/Page column 6; 36-37
