239074-29-4
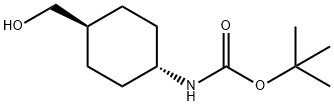 239074-29-4 结构式
239074-29-4 结构式
基本信息
反-(4-羟甲基)环己基氨基甲酸叔丁酯
(反式-4-羟甲基环己基)氨基甲酸叔丁酯
反式-1-(BOC-氨基)-4-(羟甲基)环己烷
叔-丁基 N-[反-4-(羟甲基)环己基]氨基甲酯
反式-1-(BOC-氨基)-4-(羟甲基)环己烷,97%
trans-4-(Boc-amino)cyclohexanemethanol
trans-1-(Boc-amino)-4-(hydroxymethyl)cyclohexane,97%
trans-1-(Boc-aMino)-4-(hydroxyMethyl)cyclohexane, 97%
TERT-BUTYL TRANS-(4-HYDROXYMETHYL)CYCLOHEXYLCARBAMATE
Trans tert-butyl 4-(hydroxyMethyl)cyclohexyl)carbaMate
trans-4-(tert-Butoxycarbonylamino)cyclohexane-1-methanol
tert-butyl N-[trans-4-(hydroxymethyl)cyclohexyl]carbamate
tert-butyl ((1r,4r)-4-(hydroxymethyl)cyclohexyl)carbamate
tert-Butyl trans-4-(hydroxymethyl)cyclohexylcarbamate FP160825
物理化学性质
制备方法
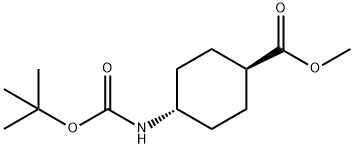
146307-51-9

239074-29-4
以反式-4-(叔丁氧羰基氨基)环己烷甲酸甲酯为原料合成反-(4-羟甲基)环己基氨基甲酸叔丁酯的一般步骤:在冰浴条件下,将LiAlH4(9.0 g,0.236 mol,1.12当量)在THF(500 mL)中的悬浮液冷却至0℃。随后,缓慢加入反式-4-(叔丁氧羰基氨基)环己烷甲酸甲酯(54.3 g,0.21 mol,1.0当量)的THF(200 mL)溶液,确保反应温度维持在10℃以下。加料完毕后,将反应混合物转移至室温搅拌过夜。反应完成后,在15℃至25℃下,用十水合硫酸钠(27 g)小心淬灭反应。通过过滤去除不溶物,浓缩滤液,得到反-(4-羟甲基)环己基氨基甲酸叔丁酯(43 g,收率89%),为白色粉末。MS-ESI:[M + 1]+:229.1。
参考文献:
[1] Patent: WO2018/67422, 2018, A1. Location in patent: Page/Page column 44; 45
[2] Patent: WO2018/69863, 2018, A1. Location in patent: Page/Page column 121-122
[3] Patent: EP2017261, 2009, A1. Location in patent: Page/Page column 30-31
[4] Patent: US2010/273841, 2010, A1. Location in patent: Page/Page column 22
[5] Patent: WO2011/92293, 2011, A2. Location in patent: Page/Page column 99-100
