24347-63-5
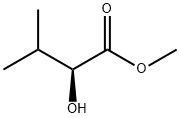 24347-63-5 结构式
24347-63-5 结构式
基本信息
(S)-Methyl 2-hydroxy-3-methylbutanoate
methyl (2s)-2-hydroxy-3-methylbutanoate
2-(S)-Hydroxy-3-methylbutyric acid methyl ester
Butanoic acid, 2-hydroxy-3-methyl-, methyl ester, (2S)-
物理化学性质
制备方法

67-56-1
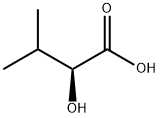
17407-55-5

24347-63-5
将甲醇(14.5 mL,360 mmol)和对甲苯磺酸一水合物(0.68 g,3.6 mmol)加入到(S)-(+)-2-羟基-3-甲基丁酸(14.20 g,120 mmol)的四氯化碳(40 mL)溶液中。将反应混合物在无水条件下回流10小时。反应完成后,将混合物冷却至室温,依次用水(15 mL)和饱和碳酸氢钠水溶液(10 mL)洗涤,用硫酸镁干燥,并在减压下浓缩。通过蒸馏纯化残余物,得到(S)-2-羟基-3-甲基丁酸甲酯(13.03 g,82%收率),为无色油状物;沸点62-63°C/15 Torr;比旋光度[α]D = +23.7(c 1.00,CHCl3),文献值[α]D = +24.3(c 1.00,CHCl3);1H NMR(CDCl3)δ 0.82(d,J = 6.9 Hz,3H,3-Me),0.98(d,J = 6.9 Hz,3H,3-Me),2.03(m,1H,3-H),2.83(d,J = 6.2 Hz,1H,OH),3.75(s,3H,CO2Me),4.01(dd,J = 6.2, 3.6 Hz,1H,2-H);13C NMR(CDCl3)δ 15.9, 18.6, 32.1, 52.2, 75.0, 175.3;IR(薄膜)νmax:3483, 1735 cm-1。
参考文献:
[1] Journal of the American Chemical Society, 1990, vol. 112, # 21, p. 7659 - 7672
[2] Tetrahedron Letters, 1989, vol. 30, # 29, p. 3757 - 3760
[3] Journal of Chemical Research, Miniprint, 1998, # 1, p. 126 - 142
[4] Heterocycles, 1999, vol. 51, # 6, p. 1321 - 1343
[5] Organic and Biomolecular Chemistry, 2005, vol. 3, # 20, p. 3749 - 3756
