24639-06-3
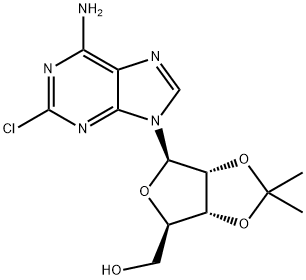 24639-06-3 结构式
24639-06-3 结构式
基本信息
((3AR,4R,6R,6AR)-6-(6-氨基-2-氯-9H-嘌呤-9-基)-2,2-二甲基四氢呋喃并[3,4-D][1,3]二氧杂环戊烯-4-基)甲醇
NSC 164687
2-Chloro-2',3'-O-isopropylideneadenosine
2-Chloro-2',3'-O-isopropylidene-D-adenosine
2-Chloro-2',3'-O-(1-Methylethylidene)adenosine
Adenosine, 2-chloro-2',3'-O-(1-methylethylidene)-
2-chloro-9-(2,3-o-isopropylidene-β-d-ribofuranosyl)adenine
2-CHLORO-9-(2,3-O-ISOPROPYLIDENE-BETA-D-RIBOFURANOSYL)ADENINE
((3AR,4R,6R,6aR)-6-(6-Amino-2-chloro-9H-purin-9-yl)-2,2-dimethyltetrahydrofuro[3,4-d][1,3]diox
((3aR,4R,6R,6aR)-6-(6-aMino-2-chloro-9H-purin-9-yl)-2,2-diMethyltetrahydrofuro[3,4-d][1,3]dioxol-4-yl)Methanol
物理化学性质
制备方法
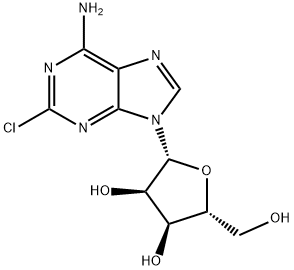
146-77-0
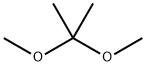
77-76-9

24639-06-3
在氮气氛围下,将2-氯腺苷(Ila,20g,66.3mmol)、2,2-二甲氧基丙烷(DMOP,60mL)和70% wt的盐酸水溶液(3mL)于室温下搅拌反应8小时。随后,缓慢加入饱和碳酸氢钠水溶液(约120mL)调节反应混合物的pH至7-9。将混合物置于冰浴中搅拌2小时后,进行过滤,用水(50mL)洗涤滤饼,随后在50℃下真空干燥6小时,得到标题化合物(式11b),纯度为99%,收率为88%。产物经1H NMR(400MHz,DMSO-d6)和13C NMR(100MHz,DMSO-d6)表征,数据如下:1H NMR δ8.36(s,1H),δ7.87(s,2H),δ6.06(d,J = 2.4Hz,1H),δ5.28(dd,J = 6Hz,2.4Hz,1H),δ5.08(t,J = 5.6Hz,1H),δ4.94(dd,J = 6Hz,2Hz,1H),δ4.21(m,1H),δ3.54(m,2H),δ1.54(s,3H),δ1.33(s,3H);13C NMR δ157.3, 153.6, 150.4, 140.4, 118.6, 113.6, 89.8, 87.2, 83.9, 81.7, 62.0, 27.5, 25.7。
参考文献:
[1] Nucleosides and Nucleotides, 1999, vol. 18, # 10, p. 2175 - 2191
[2] Patent: WO2015/85497, 2015, A1. Location in patent: Paragraph 0041-0044
[3] Bioorganic and Medicinal Chemistry Letters, 2011, vol. 21, # 15, p. 4556 - 4560
[4] Bioorganic and Medicinal Chemistry Letters, 2012, vol. 22, # 1, p. 85 - 89
[5] Journal of Medicinal Chemistry, 1990, vol. 33, # 7, p. 1919 - 1924
