247037-82-7
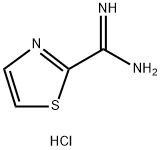 247037-82-7 结构式
247037-82-7 结构式
基本信息
2-噻唑甲咪盐酸盐
2-噻唑甲脒盐酸盐
噻唑-2-甲脒盐酸盐
2-噻唑羧酰胺盐酸盐
2-噻唑甲脒单盐酸盐
2-噻唑甲酰亚胺酰胺盐酸盐
2-Thiazolecarboxamidine
2-Thiazolecarboxamidine,HCl
2-ThiazolecarboxiMidaMide HCl
THIAZOLE-2-CARBOXIMIDAMIDE HCL
2-Thiazolecarboxamidine Hydrochloride
Thiazole-2-carboxamidine hydrochloride
2-ThiazolecarboxiMidaMide hydrochloride
Thiazole-2-carboxiMidaMide hydrochloride
2-Thiazolecarboximidamide monohydrochloride
物理化学性质
制备方法
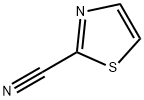
1452-16-0

247037-82-7
以2-氰基噻唑为原料合成2-噻唑甲脒盐酸盐的一般步骤:在SQL反应釜中加入甲醇(12L),随后加入2-氰基噻唑(2200g,20.00mol,1当量)和甲醇钠(54g,1mol,0.05当量)。将混合物在10℃下搅拌0.5小时。通过TLC(石油醚:乙酸乙酯 = 2:1)监测反应进程,确认原料消失并检测到中间体的生成。接着,加入氯化铵(1296g,24.00mol,1.2当量),并将混合物在65℃下搅拌16小时。再次通过TLC(石油醚:乙酸乙酯 = 2:1)确认中间体消失,表明反应完成。反应混合物经过滤除去固体氯化铵(210g),滤液经干燥得到粗产物。粗产物用乙酸乙酯(20L)浆化2小时后过滤,得到2-噻唑甲脒盐酸盐(3200g),纯度为98%。通过定量NMR测定,其有效含量为95%。分离收率为93%。1H NMR(400MHz,DMSO-d6)δ 8.26(d,J = 2.76Hz,1H),8.38(d,J = 3.01Hz,1H),9.81(br.s.,1H)。LCMS:m/z 127.9 [M + H]+。
参考文献:
[1] Journal of Medicinal Chemistry, 2017, vol. 60, # 8, p. 3352 - 3371
[2] ACS Medicinal Chemistry Letters, 2017, vol. 8, # 9, p. 969 - 974
[3] Patent: US2018/312512, 2018, A1. Location in patent: Paragraph 0091; 0092; 0100; 0101; 0102; 0103
[4] Bioorganic and Medicinal Chemistry Letters, 2010, vol. 20, # 1, p. 299 - 301
[5] Patent: WO2014/37480, 2014, A1. Location in patent: Page/Page column 91; 92
