2614-06-4
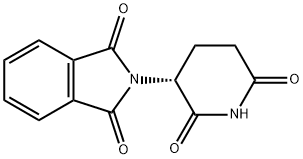 2614-06-4 结构式
2614-06-4 结构式
基本信息
酞咪呱啶酮(反应停)
(R)-(+)-THALIDOMIDE
6-dioxo-3-piperidyl)-n-(d-(+)-phthalimid
N-[(R)-2,6-Dioxopiperidine-3-yl]phthalimide
(+)-N-[(R)-2,6-Dioxo-3-piperidinyl]phthalimide
6-dioxo-3-piperidinyl)-3(2h)-dion(r)-1h-isoindole-2-(2
(3R)-3-(1,3-Dioxo-2H-isoindole-2-yl)piperidine-2,6-dione
(R)-2-(2,6-DIOXO-3-PIPERIDINYL)-1H-ISOINDOLE-1,3-(2H)-DIONE
R-(+)-2-(2,6-DIOXO-3-PIPERIDINYL)-1H-ISOINDOLE-1,3(2H)-DIONE
(+)-2-[(R)-2,6-Dioxo-3-piperidinyl]-1H-isoindole-1,3(2H)-dione
物理化学性质
安全数据
toxic compounds or compounds which causing chronic effects
Repr. 1B
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | HY-14658B | (+)-沙利度胺 (R)-Thalidomide | 2614-06-4 | 1 mg | 640元 |
| 2025/12/22 | HY-14658B | (+)-沙利度胺 (R)-Thalidomide | 2614-06-4 | 5 mg | 1440元 |
| 2025/12/22 | HY-14658B | (+)-沙利度胺 (R)-Thalidomide | 2614-06-4 | 10 mg | 2240元 |
常见问题列表
The transport of the (R)-Thalidomide from the R-imprinted MIP-1 through the donor phase to the receiver phase is much less owing to the stronger retention of the thalidomide in the organic phase. With the affinity of (R)-Thalidomide by the MIP present surface capture, that is more strongly than the other forms. In the case of (R)-Thalidomide, it is found to bind to the selective sites of the MIP more strongly than the other which reflects their different biological entities.
The (S)-Thalidomide imprints MIP nanoparticles exerted a greater cytotoxic effect on the caco-2 cells than the (R)-Thalidomide imprinted MIPs.
Adult female F344 rats are implanted with 9L gliosarcoma tumours intracranially, subcutaneously (flank), or both. Effectiveness of oral thalidomide alone, and with intraperitoneal BCNU or cisplatin combination chemotherapy, is assessed after several weeks treatment. Both serum and tissue concentrations of (R)-thalidomide are 40-50% greater than those of (S)-thalidomide. Co-administration of BCNU or cisplatin with thalidomide did not alter the concentration enantioselectivity.

