2717-76-2
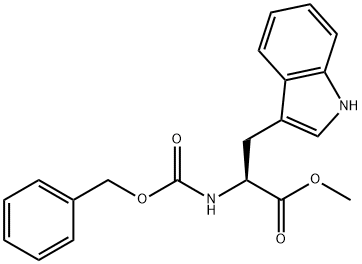 2717-76-2 结构式
2717-76-2 结构式
基本信息
CBZ-L-色氨酸甲酯
N-CBZ-L-色氨酸甲酯
N-苄氧羰基-L-色氨酸甲酯
(S)-2-(((苄氧基)羰基)氨基)-3-(1H-吲哚-3-基)丙酸甲酯
Cbz-L-Trp-OMe
Z-TRP-OME USP/EP/BP
Cbz-L-Tryptophan-methyl ester
Z-L-tryptophan beta-Methyl ester
N-Carbobenzoxy-L-tryptophan methyl ester
L-Tryptophan, N-[(phenylmethoxy)carbonyl]-, methyl ester
(S)-methyl 2-(benzyloxycarbonylamino)-3-(1H-indol-3-yl)propanoate
methyl (2S)-2-{[(benzyloxy)carbonyl]amino}-3-(1H-indol-3-yl)propanoate
物理化学性质
制备方法

67-56-1
7432-21-5

2717-76-2
在氮气保护下,将(S)-N-苄氧基羰基-L-色氨酸(2.45 g, 7.2 mmol)悬浮于10 mL无水二氯甲烷中,加入4-二甲氨基吡啶(DMAP, 0.1 g, 0.82 mmol)和甲醇(0.28 g, 11.8 mmol)。将反应混合物冷却至0℃,缓慢滴加溶解于二氯甲烷的N,N'-二环己基碳二亚胺(DCC)溶液。滴加完毕后,将反应体系逐渐升温至室温并持续搅拌12小时。反应完成后,通过过滤去除不溶物,滤液经减压浓缩。粗产物通过快速柱色谱法(洗脱剂:正己烷/乙酸乙酯,4:1至2:1梯度洗脱)纯化,得到目标化合物(S)-2-(((苄氧基)羰基)氨基)-3-(1H-吲哚-3-基)丙酸甲酯(2.5 g, 98%)为无色油状物。薄层色谱(TLC)Rf = 0.19(展开剂:乙酸乙酯/正己烷=1:2)。核磁共振氢谱(1H NMR, 300 MHz, CDCl3):δ 8.94(宽峰, 1H), 7.49(d, J = 7.7 Hz, 1H), 7.25-6.98(多重峰, 9H), 6.81(单峰, 1H), 5.84(d, J = 8.7 Hz, 1H), 4.98(d, J = 3.2 Hz, 2H), 4.68(四重峰, J = 7.3 Hz, 1H), 3.25(单峰, 3H), 3.21(多重峰, 2H)。核磁共振碳谱(13C NMR, 75 MHz, CDCl3):δ 172.2, 155.9, 141.8, 136.8, 134.9, 128.6, 127.8, 126.9, 123.4, 122.3, 120.0, 118.4, 112.1, 109.4, 67.0, 65.1, 55.1。傅里叶变换红外光谱(FTIR, KBr, 纯样):ν 1707, 1508, 1456, 1436, 1215, 742 cm-1。高分辨质谱(HRMS, EI):计算值C20H20O4N2 [M]+ 352.1418,实测值352.1425。
参考文献:
[1] Organic and Biomolecular Chemistry, 2003, vol. 1, # 20, p. 3492 - 3494
[2] Advanced Synthesis and Catalysis, 2010, vol. 352, # 7, p. 1107 - 1112
[3] Patent: US2011/269972, 2011, A1. Location in patent: Page/Page column 18-19
