29840-56-0
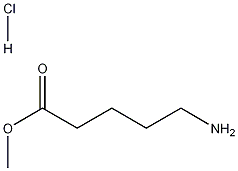 29840-56-0 结构式
29840-56-0 结构式
基本信息
5-氨基戊酸甲酯盐酸盐
Methyl 5-aminovalerate hydrochloride
Ethyl 5-aMinopentanoate hydrochloride
Methyl 5-aminopentanoate hydrochloride
5-Aminopentanoic acid methyl ester hydrochloride
delta-Aminovaleric acid methyl ester hydrochloride
methyl ester- 5-amino- Pentanoic acid, hydrochloride (1:1)
物理化学性质
制备方法

67-56-1
660-88-8

29840-56-0
将5-氨基戊酸(22.45 mmol,1.0 equiv)溶解于20-30 mL无水甲醇中,置于配备回流冷凝器的双颈烧瓶中。在5-10分钟内缓慢滴加亚硫酰氯(31.43 mmol,1.4 equiv)。滴加完毕后,将反应混合物加热回流3小时。随后,将混合物在室温下搅拌过夜。反应完成后,通过旋转蒸发仪(RVE)除去溶剂。得到的白色或浅黄色固体用正己烷(3×50 mL)洗涤,并用另一部分正己烷进行蒸发。产物在低压下干燥,得到5-氨基戊酸甲酯盐酸盐(2a)为半透明固体,产量3.46 g(收率92%)。熔点:141-143°C(文献值146.3°C [42]);1H NMR(300 MHz, D2O):δ = 3.57(s, 3H, COOCH3),2.88(t, 2H, J = 7.0 Hz, C5-H2),2.30-2.35(m, 2H, C2-H2),1.53-1.59(m, 4H, C3-H2和C4-H2)ppm;13C NMR(75 MHz, D2O):δ = 179.2(C1),54.7(COOCH3),41.6(C5),35.5(C2),28.6(C4),23.6(C3)ppm;IR(固体):ν = 3021(s),2945(s),1735(s),1596(m),1576(m),1519(s),1474(w),1444(w),1414(w),1346(w),1271(m),1187(s),1152(m),1068(w),1055(m),984(w),952(m),899(w),882(w),865(w),748(s),651(w)cm-1。
参考文献:
[1] Journal of Medicinal Chemistry, 2006, vol. 49, # 20, p. 6094 - 6103
[2] Bioorganic and Medicinal Chemistry, 2017, vol. 25, # 10, p. 2730 - 2742
[3] Angewandte Chemie - International Edition, 2013, vol. 52, # 28, p. 7228 - 7232
[4] Angew. Chem., 2013, vol. 125, # 28, p. 7369 - 7373,5
[5] Organic and Biomolecular Chemistry, 2016, vol. 14, # 25, p. 6010 - 6023
常见问题列表
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | XW022984057104 | 5-氨基戊酸乙酯盐酸盐 Ethyl 5-aminopentanoate hydrochloride | 29840-56-0 | 5g | 152元 |
| 2025/12/22 | XW022984057103 | 5-氨基戊酸乙酯盐酸盐 Ethyl 5-aminopentanoate hydrochloride | 29840-56-0 | 1g | 53元 |
| 2025/12/22 | XW022984057102 | 5-氨基戊酸乙酯盐酸盐 Ethyl 5-aminopentanoate hydrochloride | 29840-56-0 | 250mg | 34元 |
