31140-42-8
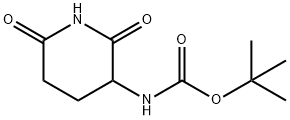 31140-42-8 结构式
31140-42-8 结构式
基本信息
3-BOC-氨基-2,6-哌啶酮
3-BOC-氨基-2,6-二氧哌啶
3-BOC-氨基-2,6-二氧代哌啶
3-N-叔丁氧羰基氨基-2,6-二氧代哌啶
N-(2,6-二氧代哌啶-3-基)氨基甲酸叔丁酯
3-N-叔丁氧羰基氨基-2,6-二氧代哌啶(泊马度胺中间体)
3-N-叔丁氧羰基氨基-2,6-二氧代哌啶(泊马度胺中间体) 1KG
Pomalidomide/lenalidomide INT I
3-BOC-AMINO-2,6-DIOXOPIPERIDINE
TERT-BUTYL 2,6-DIOXOPIPERIDIN-3-YLCARBAMATE
tert-Butyl N-(2,6-dioxopiperidin-3-yl)carbamate
2,6-Dioxo-3-piperidinecarbamic acid tert-butyl ester
1-amino-2,6-dioxo-3-piperidinecarboxylic acid tert-butyl ester
CarbaMic acid, (2,6-dioxo-3-piperidinyl)-, 1,1-diMethylethyl ester
Carbamic acid, N-(2,6-dioxo-3-piperidinyl)-, 1,1-dimethylethyl ester
物理化学性质
制备方法
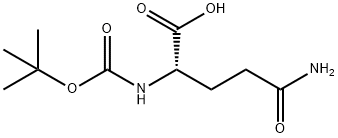
13726-85-7

31140-42-8
以BOC-L-谷氨酰胺为原料合成3-Boc-氨基-2,6-二氧代哌啶的一般步骤:将N-(叔丁氧基羰基)-L-谷氨酰胺(4.92 g, 20 mmol)和羰基二咪唑(1.70 g, 10.5 mmol)溶解于四氢呋喃(100 mL)中,加热回流反应9小时。反应完成后,减压蒸馏除去溶剂,得到粗产物。粗产物用热乙酸乙酯重结晶,得到目标化合物3-Boc-氨基-2,6-二氧代哌啶(2.04 g,收率45%),为白色晶体。熔点:214-215℃;1H NMR(DMSO-d6)δ 4.22(dd,J = 6.2 Hz,J = 11.0 Hz,1H),2.77-2.65(m,1H),2.45(m,1H),1.96-1.87(m,2H),1.40(s,9H);质谱(CI/CH4)m/z 227 [M-1]+。
参考文献:
[1] Organic Letters, 2003, vol. 5, # 16, p. 2865 - 2867
[2] Bioorganic and Medicinal Chemistry Letters, 2007, vol. 17, # 21, p. 5819 - 5824
[3] Bioorganic and Medicinal Chemistry, 2010, vol. 18, # 2, p. 650 - 662
[4] Patent: WO2005/28436, 2005, A2. Location in patent: Page/Page column 30; 53
[5] Bioorganic and Medicinal Chemistry Letters, 2016, vol. 26, # 21, p. 5260 - 5262
