31608-22-7
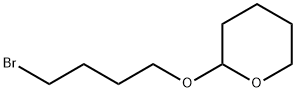 31608-22-7 结构式
31608-22-7 结构式
基本信息
2-(4-溴丁氧基)四氢-2H-吡喃
2H-Pyran, 2-(4-broMobutoxy)tetrahydro-
2-(4-Bromobutyloxy)tetrahydro-2H-pyran
2-(4-Bromobutoxy)tetrahydro-2H-pyran >
2-(4-Bromobutoxy)tetrahydro-2H-pyran
1-Bromo-4-(tetrahydro-2H-pyran-2-yloxy)butane
制备方法
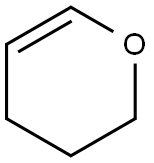
110-87-2

33036-62-3

31608-22-7
以3,4-二氢-2H-吡喃和4-溴-1-丁醇为原料合成2-(4-溴丁氧基)四氢-2H-吡喃的一般步骤:在0℃下,将3,4-二氢-2H-吡喃(8.0g,95.36mmol)缓慢加入至4-溴-1-丁醇(12.0g,79.47mmol)的二氯甲烷(150mL)溶液中,随后加入对甲苯磺酸(20mg)作为催化剂。反应1小时后,用饱和NaHCO3溶液(5mL)小心淬灭反应,随后依次用水(100mL)和盐水(70mL)洗涤有机层。将有机层真空浓缩,得到粗产物。通过硅胶柱色谱法纯化残余物,使用2%乙酸乙酯/己烷作为洗脱剂,得到2-(4-溴丁氧基)四氢-2H-吡喃(16.57g,收率88%)为无色油状物。薄层色谱(TLC)分析条件:10%乙酸乙酯/己烷,Rf值0.50。1H NMR(CDCl3, 300MHz)δ 4.58(t, J = 2.5Hz, 1H),3.90-3.72(m, 2H),3.38-3.50(m, 4H),1.92-2.04(m, 2H),1.65-1.80(m, 4H),1.50-1.60(m, 4H)。
参考文献:
[1] European Journal of Organic Chemistry, 2002, # 17, p. 3024 - 3033
[2] Journal of Organic Chemistry, 1986, vol. 51, # 18, p. 3553 - 3555
[3] Journal of Organic Chemistry, 1993, vol. 58, # 22, p. 5964 - 5966
[4] Bulletin de la Societe Chimique de France, 1994, vol. 131, # 6, p. 699 - 705
[5] Patent: WO2004/4706, 2004, A1. Location in patent: Preparation 1
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | XW023160822704 | 2-(4-溴丁氧基)四氢-2H-吡喃 2-(4-Bromobutoxy)tetrahydro-2H-pyran | 31608-22-7 | 5g | 1681元 |
| 2025/12/22 | XW023160822703 | 2-(4-溴丁氧基)四氢-2H-吡喃 2-(4-Bromobutoxy)tetrahydro-2H-pyran | 31608-22-7 | 1g | 477元 |
| 2025/12/22 | XW023160822702 | 2-(4-溴丁氧基)四氢-2H-吡喃 2-(4-Bromobutoxy)tetrahydro-2H-pyran | 31608-22-7 | 250mg | 318元 |
