31949-21-0
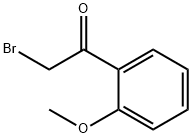 31949-21-0 结构式
31949-21-0 结构式
基本信息
α-溴-2'-甲氧基苯乙酮
2-溴-2'-甲氧基苯乙酮
Α-溴-2'-甲氧基苯乙酮
2-BROMO-2'-METHOXYACETOPHENONE
2'-METHOXYPHENACYL BROMIDE
2-METHOXYPHENACYL BROMIDE
A-BROMO-O-METHOXYACETOPHENONE
AKOS BBS-00003867
O-METHOXYPHENACYL BROMIDE
alpha-Bromo-o-methoxyacetophenone
Ethanone, 2-bromo-1-(2-methoxyphenyl)-
alpha-Bromo-2'-methoxyacetophenone
alpha-Bromo-2-methoxyacetophenone
bromomethyl 2-methoxyphenyl ketone
2-Methoxyphenacyl bromide 98%
2''-METHOXY-2-BROMOACETOPHENONE
2-BROMO-2''-METHOXYACETOPHENONE 98%
α-Bromo-2'-methoxyacetophenone
1-(2-Methoxyphenyl)-2-bromoethanone
1-(Bromoacetyl)-2-methoxybenzene
2'-Methoxy-α-bromoacetophenone
物理化学性质
安全数据
制备方法
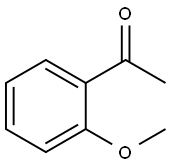
579-74-8

31949-21-0
以2'-甲氧基苯乙酮为原料合成邻甲氧基-2-溴苯乙酮的一般步骤如下:将溴化铜(II)(2.97 g,6.66 mmol)加入装有回流冷凝器的双颈圆底烧瓶中。向烧瓶中加入乙酸乙酯(10.0 mL),在氮气保护下,将混合物于70℃搅拌5分钟。缓慢加入1-(2-甲氧基苯基)乙酮(0.50 g,3.33 mmol)的氯仿(10.0 mL)溶液,随后将反应混合物回流8小时。反应完成后,冷却至室温,通过硅藻土垫过滤,并用乙酸乙酯(20 mL)洗涤滤饼。将滤液减压浓缩,得到的粗产物通过柱色谱法(洗脱剂比例为乙酸乙酯:己烷 = 1:4)进行纯化,得到纯品邻甲氧基-2-溴苯乙酮(0.73 g,收率96%),为棕色液体。薄层色谱Rf值为0.43(展开剂比例为乙酸乙酯:己烷 = 1:4);1H NMR(300 MHz, CDCl3)δ: 7.81(1H, dd, J = 7.8, 1.8 Hz),7.52(1H, td, J = 7.8, 1.8 Hz),7.05-6.96(2H, m),4.61(2H, s),3.94(3H, s);13C NMR(75 MHz, CDCl3)δ: 192.3, 158.8, 134.9, 131.6, 124.9, 121.2, 111.7, 56.0, 38.0。
参考文献:
[1] Bulletin of the Korean Chemical Society, 2017, vol. 38, # 12, p. 1481 - 1485
[2] Tetrahedron Letters, 2006, vol. 47, # 27, p. 4707 - 4710
[3] Tetrahedron Letters, 2009, vol. 50, # 6, p. 700 - 703
[4] Chemistry - A European Journal, 2011, vol. 17, # 28, p. 7953 - 7959
[5] Molecules, 2017, vol. 22, # 5,
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | XW023194921004 | 2-溴-2''-甲氧基苯乙酮 2-Bromo-1-(2-methoxyphenyl)ethanone | 31949-21-0 | 100g | 1166元 |
| 2025/12/22 | XW023194921003 | 2-溴-2''-甲氧基苯乙酮 2-Bromo-1-(2-methoxyphenyl)ethanone | 31949-21-0 | 25g | 294元 |
| 2025/12/22 | XW023194921002 | 2-溴-2''-甲氧基苯乙酮 2-Bromo-1-(2-methoxyphenyl)ethanone | 31949-21-0 | 10g | 120元 |
