3392-12-9
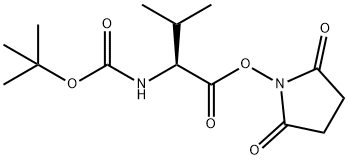 3392-12-9 结构式
3392-12-9 结构式
基本信息
BOC-L-缬氨酸羟基琥珀酰亚胺酯
N-叔丁氧羰基-L-缬氨酸羟基琥珀酰亚胺酯
BOC-L-VALINE N-HYDROXYSUCCINIMIDE ESTER
BOC-VALINE-OSU
BOC-VAL-OSU
N-ALPHA-T-BOC-L-VALINE N-HYDROXYSUCCINIMIDE ESTER
N-BOC-L-VAL-N-HYDROXYSUCCINIMIDE
N-TERT-BUTOXYCARBONYL-L-VALINE-N-HYDROXYSUCCINIMIDE ESTER
tert-butyl (S)-[1-[[(2,5-dioxopyrrolidin-1-yl)oxy]carbonyl]-2-methylpropyl]carbamate
BOC-L-VALINE HYDROXYSUCCINIMIDESTER
N-(tert-butoxycarbonyl)-L-valine hydroxysuccinimide ester
tert-Butoxycarbonyl-L-valine N-hydroxysuccinimide ester
[(S)-1-[[(2,5-Dioxo-1-pyrrolidinyl)oxy]carbonyl]-2-methylpropyl]carbamic acid 1,1-dimethylethyl ester
Boc-L-Val-ONSu
N-(tert-Butoxycarbonyl)-L-valine succinimidyl ester
物理化学性质
制备方法
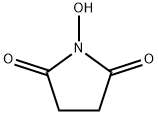
6066-82-6
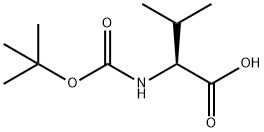
13734-41-3

3392-12-9
步骤A:在干燥的圆底烧瓶中依次加入Boc-L-缬氨酸(1.03 g,4.74 mmol)、N-羟基琥珀酰亚胺(1.22 g,10.6 mmol)和1-乙基-3-(3-二甲氨基丙基)碳二亚胺盐酸盐(EDC,1.60 g,8.35 mmol)。将上述试剂溶于无水二氯甲烷(30 mL)中,反应体系通过橡胶隔膜密封,并用氩气置换三次以排除氧气。随后,将反应混合物在室温下搅拌反应。3天后,薄层色谱(TLC)检测(茚三酮显色)确认Boc-L-缬氨酸已完全反应。反应液依次用水、饱和碳酸氢钠水溶液洗涤,水相用二氯甲烷反萃取。合并有机相,用饱和食盐水洗涤,无水硫酸钠干燥,过滤。滤液经减压浓缩后,真空干燥得到白色固体Boc-L-缬氨酸羟基琥珀酰亚胺酯(1.52 g,产率100%)。产物经1H-NMR(300 MHz,CDCl3)表征:δ 4.98(宽峰,1H),4.58(双峰,1H),2.82(多重峰,4H),2.27(多重峰,1H),1.44(单峰,9H),1.03(双峰,6H)。
参考文献:
[1] Patent: WO2016/160615, 2016, A1. Location in patent: Paragraph 0444; 0445
[2] Journal of Medicinal Chemistry, 1998, vol. 41, # 14, p. 2461 - 2480
[3] Journal of Medicinal Chemistry, 1991, vol. 34, # 9, p. 2852 - 2857
[4] Journal of the Chemical Society, Dalton Transactions: Inorganic Chemistry (1972-1999), 1984, p. 2305 - 2308
[5] Journal of Organic Chemistry, 1986, vol. 51, # 24, p. 4580 - 4585
