360773-84-8
中文名称
1-(4-溴苯基)-环丙基]氨基甲酸叔丁酯
英文名称
t-Butyl 1-(4-bromophenyl)cyclopropylcarbamate
CAS
360773-84-8
分子式
C14H18BrNO2
分子量
312.2
MOL 文件
360773-84-8.mol
更新日期
2026/02/02 09:02:14
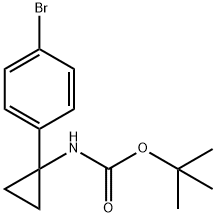 360773-84-8 结构式
360773-84-8 结构式
基本信息
中文别名
2-氟-32-(甲硫基)吡啶叔丁氧羰基-1-(4-溴)环丙基胺
N-BOC-1-(4-溴苯基)环丙胺
[1-(4-溴苯基)-环丙基]氨基甲酸叔丁酯
1-(N-BOC-氨基)-1-(4-溴苯基)环丙烷
英文别名
tert-Butyl (1-(4-broMophenyl)cyclopropyl)carbaMatetert-Butyl N-[1-(4-bromophenyl)cyclopropyl]carbamate
tert-Butyl (1-(4-bromophenyl)
2-Fluoro-32-(methylthio)pyridine
N-Boc-1-(4-bromophenyl)cyclopropanamine
t-Butyl 1-(4-bromophenyl)cyclopropylcarbamate
1-(N-BOC-Amino)-1-(4-bromophenyl)cyclopropane
[1-(4-Bromo-phenyl)-cyclopropyl]-carbamic acid tert-butyl
[1-(4-Bromo-phenyl)-cyclopropyl]-carbamic acid tert-butyl ester
Carbamic acid, N-[1-(4-bromophenyl)cyclopropyl]-, 1,1-dimethylethyl ester
所属类别
有机原料:羧酸酯及其衍生物物理化学性质
熔点126-128°
沸点385.2±31.0 °C(Predicted)
密度1.37±0.1 g/cm3(Predicted)
储存条件Sealed in dry,Room Temperature
酸度系数(pKa)11.94±0.20(Predicted)
外观White to off-white Solid
InChIInChI=1S/C14H18BrNO2/c1-13(2,3)18-12(17)16-14(8-9-14)10-4-6-11(15)7-5-10/h4-7H,8-9H2,1-3H3,(H,16,17)
InChIKeyZJEMFURAGDYBTG-UHFFFAOYSA-N
SMILESC(OC(C)(C)C)(=O)NC1(C2=CC=C(Br)C=C2)CC1
制备方法
方法1

24424-99-5
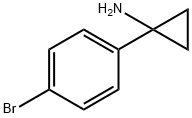
345965-54-0
![1-(4-溴苯基)-环丙基]氨基甲酸叔丁酯](/CAS2/GIF/360773-84-8.gif)
360773-84-8
以1-(4-溴苯基)环丙胺和二碳酸二叔丁酯为原料,合成[1-(4-溴苯基)-环丙基]氨基甲酸叔丁酯的一般步骤如下:在100-mL圆底烧瓶中,将1-(4-溴苯基)环丙烷-1-胺(1g,4.48mmol,1.00当量)和二碳酸二叔丁酯(3.1g,13.49mmol,3.01当量)溶于四氢呋喃(20mL)中。随后,在室温下向反应体系中缓慢加入碳酸氢钠(5g,59.5mmol,13.29当量)的水溶液(10mL)。反应混合物在室温下搅拌过夜。反应完成后,用乙酸乙酯(3×10mL)萃取反应混合物,合并有机层,用无水硫酸钠干燥,减压浓缩除去溶剂。残余物通过硅胶柱色谱纯化,采用乙酸乙酯/石油醚(10%至50%梯度)作为洗脱剂,得到[1-(4-溴苯基)-环丙基]氨基甲酸叔丁酯(1.47g,99%),为浅黄色固体。质谱(MS)分析显示:m/z = 255.7 [M+H-56]+。
参考文献:
[1] Patent: US2016/96834, 2016, A1. Location in patent: Paragraph 0390
[2] Patent: US2012/108574, 2012, A1. Location in patent: Page/Page column 113
[3] Patent: WO2016/57762, 2016, A1. Location in patent: Page/Page column 30
[4] Patent: WO2016/102672, 2016, A2. Location in patent: Page/Page column 115