367-29-3
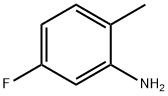 367-29-3 结构式
367-29-3 结构式
基本信息
2-METHYL-5-FLUOROANILINE
3-FLUORO-6-METHYLANILINE
5-FLUORO-2-METHYLANILINE
5-FLUORO-O-TOLUIDINE
TIMTEC-BB SBB004266
Benzenamine, 5-fluoro-2-methyl-
2-Amino-4-fluorotoluene~5-Fluoro-o-toluidine
4-Fluoro-2-Aminotoluene
Fluoromethylaniline7
5-Fluoro-2-methylaniline,98+%
5-Fluoro-2-methylaniline 98%
5-Fluoro-o-toluidine (NH2=1)
5-Fluoro-2-toluidine
物理化学性质
安全数据
Eye Irrit. 2
Skin Irrit. 2
STOT SE 3
制备方法
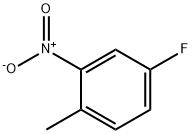
446-10-6

367-29-3
5-氟-2-甲基苯胺的合成:将4-氟-1-甲基-2-硝基苯(2.50 g,16 mmol)溶于乙醇(50 mL)中。在0℃下,向该溶液中依次加入铁粉(4.50 g,81 mmol)和0.25 mL盐酸,将反应混合物回流12小时。反应完成后,将混合物冷却至室温,用乙酸乙酯稀释,通过硅藻土(Celite)过滤,并用乙酸乙酯洗涤滤饼。合并的滤液用碳酸氢钠溶液碱化后,依次用水和盐水洗涤有机层。有机层经无水硫酸钠干燥后,减压浓缩得到粗产物。粗产物通过柱色谱法(正己烷/乙酸乙酯,1:1)纯化,得到标题化合物(1.4 g,70%)为浅棕色固体。1H NMR (400 MHz, DMSO-d6): δ 6.87 (t, J = 7.6 Hz, 1H), 6.38-6.34 (m, 1H), 6.22-6.18 (m, 1H), 5.11 (bs, 2H), 1.99 (s, 3H). MS (ES) m/e: 126 (M + 1)+.
参考文献:
[1] Journal of Organic Chemistry, 1992, vol. 57, # 19, p. 5254 - 5255
[2] Patent: WO2015/104662, 2015, A1. Location in patent: Page/Page column 37
[3] Patent: US2016/326151, 2016, A1. Location in patent: Paragraph 0204
[4] Journal of the American Chemical Society, 1948, vol. 70, p. 439
[5] Journal of the Chemical Society, 1953, p. 3326
知名试剂公司产品信息
5-Fluoro-2-methylaniline, 99%(367-29-3)
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2026/03/03 | B25381 | 5-氟-2-甲基苯胺, 98% 5-Fluoro-2-methylaniline, 98% | 367-29-3 | 10g | 423元 |
| 2025/12/22 | F0232 | 5-氟-2-甲苯胺 5-Fluoro-2-methylaniline | 367-29-3 | 5G | 115元 |
| 2025/12/22 | XW03672932 | 5-氟-2-甲基苯胺 5-fluoro-o-toluidine;5-fluoro-2-methylaniline;2-amino-4-fluorotoluene;2-methyl-5-fluoroaniline | 367-29-3 | 25G | 52元 |


