37674-72-9
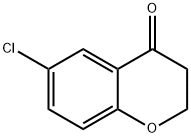 37674-72-9 结构式
37674-72-9 结构式
基本信息
6-氯苯并二氢吡喃-4-酮
6-CHLORO-4-CHROMANONE
6-CHLOROCHROMAN-4-ONE
6-CHLOROCHROMANONE
BUTTPARK 35\07-32
TIMTEC-BB SBB003732
4H-1-Benzopyran-4-one, 6-chloro-2,3-dihydro-
6-Chloro-4-chromanone (6-Chlorochroman-4-one)
6-Chloro-2,3-dihydro-4H-1-benzopyran-4-one
6-Chloro-3,4-dihydro-2H-1-benzopyran-4-one
物理化学性质
制备方法
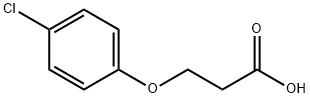
3284-79-5

37674-72-9
以3-(对氯苯氧基)丙酸为原料合成6-氯苯并二氢吡喃-4-酮的一般步骤:将3-(对氯苯氧基)丙酸(2g,10mmol)与浓硫酸(20mL)在室温下搅拌反应1小时。反应完成后,将混合物倒入冰水中,并用乙酸乙酯萃取。有机层用饱和碳酸氢钠水溶液洗涤两次,随后用硫酸镁干燥。干燥后的有机相经过滤,并在减压下浓缩。粗产物通过乙酸乙酯/己烷(1:5)混合溶剂重结晶纯化。过滤收集所得晶体,用己烷洗涤,干燥后得到目标产物6-氯苯并二氢吡喃-4-酮(1g,产率55%):熔点98-101℃(文献值:100-101℃);1H NMR(DMSO-d6)δ2.82(t,J=6.5Hz,2H,3-H2),4.56(t,J=6.5Hz,2H,2-H2),7.1(d,J=8.9Hz,1H,8-H),7.6(dd,J=8.9Hz/2.7Hz,1H,7-H),7.68(d,J=2.7Hz,1H,5-H);13C NMR(DMSO-d6)δ36.8(C-3),66.9(C-2),120.3(C-8),122.0(C-4a/C-6),125.3(C-5),135.6(C-7),160.1(C-8a),190.6(C-4)。
参考文献:
[1] Chinese Journal of Chemistry, 2011, vol. 29, # 4, p. 757 - 764
[2] European Journal of Medicinal Chemistry, 2012, vol. 54, p. 834 - 844
[3] Journal of the Indian Chemical Society, 1939, vol. 16, p. 639,644
[4] Journal of the Indian Chemical Society, 1957, vol. 34, p. 467,468
[5] Journal of the American Chemical Society, 1954, vol. 76, p. 5065,5067
