37763-23-8
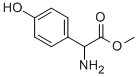 37763-23-8 结构式
37763-23-8 结构式
基本信息
(R)-对羟基苯甘氨酸甲酯
D-(-)-对羟基本甘氨酸甲酯
甲基(R)-2-氨基-2-(4-羟苯基)乙酸酯
METHYLD-(一)-4-HYCDROXY-PHENYLGLYCINATE
CEFADROXIL IMPURITY 1 (METHYL (2R)-2-AMINO-2-(4-HYDROXYPHENYL)ACETATE)
D- methyl p-hydroxyphenylglycine
METHYL DL-4-HYDROXYPHENYLGLYCINE HCL
Methyl D-(-)-4-hydroxy-phenylglycine
D-4-Hydroxyphenylglycine methyl ester
4-Hydroxy-D-phenylglycine methyl ester
METHYL D-(-)-4-HYDROXY-PHENYLGLYCINATE
methyl (R)-amino(4-hydroxyphenyl)acetate
(R)-α-(p-Hydroxyphenyl)glycine methyl ester
Methyl D-(-)-4-hydroxyphenylglycinate, 95+%
物理化学性质
制备方法

67-56-1
22818-40-2

37763-23-8
步骤1:将(R)-2-氨基-2-(4-羟基苯基)乙酸(D-4-羟基苯基甘氨酸,10.0 g,60.1 mmol)溶于甲醇(200 mL)中,缓慢滴加亚硫酰氯(8 mL),室温下搅拌反应10小时。反应完成后,将混合物减压浓缩,残余物用乙醚洗涤两次,得到(R)-2-氨基-2-(4-羟基苯基)乙酸甲酯(D-4-羟基苯基甘氨酸甲酯,13.0 g,60.0 mmol,收率100%)白色固体。1H NMR (300 MHz, DMSO-d6) δ 3.68 (s, 3H), 5.07 (s, 1H), 6.85 (d, J = 8.5 Hz, 2H), 7.29 (d, J = 8.6 Hz, 2H), 9.03 (s, 3H), 10.02 (s, 1H)。步骤2:将(R)-2-氨基-2-(4-羟基苯基)乙酸甲酯(D-4-羟基苯基甘氨酸甲酯,0.302 g,1.39 mmol)溶于新制备的乙醛酸(1.27 g,13.8 mmol)和硫酸铜(II)五水合物(0.35 g,1.40 mmol)的2.5 M吡啶缓冲液和0.5 M乙酸水溶液中,室温搅拌10小时。反应液用二氯甲烷(3 × 10 mL)萃取,合并有机层,用0.5 M HCl(3 × 20 mL)洗涤,干燥后过滤,减压浓缩。残余物通过快速柱色谱纯化(硅胶,氯仿洗脱),得到2-(4-羟基苯基)-2-氧代乙酸甲酯(0.109 g,6.06 mmol,收率44%)为透明油状物。1H NMR (300 MHz, CDCl3) δ 3.99 (s, 3H), 5.41 (br s, 1H), 6.95 (d, J = 8.8 Hz, 2H), 8.00 (d, J = 8.8 Hz, 2H)。
参考文献:
[1] Journal of Medicinal Chemistry, 2002, vol. 45, # 18, p. 3946 - 3952
[2] Patent: WO2013/33093, 2013, A1. Location in patent: Page/Page column 137; 138
[3] Angewandte Chemie - International Edition, 1997, vol. 37, # 19, p. 2708 - 2714
[4] Organic Letters, 2015, vol. 17, # 4, p. 1018 - 1021
[5] Journal of the American Chemical Society, 1978, vol. 100, p. 4555 - 4568