387827-19-2
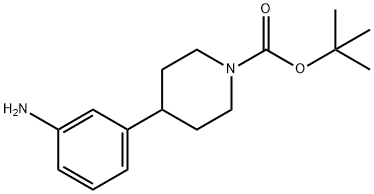 387827-19-2 结构式
387827-19-2 结构式
基本信息
4-(3-氨基苯基)-哌啶-1-羧酸叔丁酯
叔-丁基 4-(3-氨基苯基)哌啶-1-甲酸基酯
4-(3-氨基-苯基)-哌啶-1-甲酸 叔丁基 酯
tert-Butyl 4-(3-aminophenyl)
N-BOC-4-(3-AMINOPHENYL) PIPERIDINE
TERT-BUTYL 4-(3-AMINOPHENYL)PIPERIDINE-1-CARBOXYLATE
Tert-butyl 4-[3-(amino)phenyl]-1-Piperidinecarboxylate
4-(3-AMINO-PHENYL)-PIPERIDINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
1-Piperidinecarboxylic acid, 4-(3-aMinophenyl)-, 1,1-diMethylethyl ester
物理化学性质
制备方法

387827-18-1

387827-19-2
以4-(3-氨基苯基)-5,6-二氢吡啶-1(2H)-羧酸叔丁酯(3.10 g,11.3 mmol)为原料,与10% Pd/C(1.00 g)在乙醇(100 mL)中混合。反应体系采用气球法在室温下氢化2天。反应完成后,将混合物通过硅藻土过滤,并用乙醇洗涤滤饼。合并乙醇萃取液,减压浓缩。残余物通过硅胶柱色谱纯化,洗脱剂为二氯甲烷:甲醇:异丙胺(95:5:1,v/v/v),得到目标产物4-(3-氨基苯基)-哌啶-1-羧酸叔丁酯(2.63 g,收率84%)。产物经1H NMR(400 MHz,CDCl3)表征:δ 7.10(t,1H,J = 7.6 Hz),6.62(d,1H,J = 8.4 Hz),6.60-6.59(m,2H),4.27-4.18(m,2H),3.62-3.58(m,2H),2.80-2.72(m,2H),2.62-2.59(m,1H),1.89-1.52(m,4H),1.49(s,9H);ESMS m/e:277.2(M + H)+。
参考文献:
[1] Patent: US2004/38855, 2004, A1
[2] Patent: WO2004/5257, 2004, A1. Location in patent: Page 54
[3] Patent: US6727264, 2004, B1. Location in patent: Page column 56; 91
[4] Patent: US2005/154020, 2005, A1. Location in patent: Page/Page column 8
[5] Patent: US2005/154022, 2005, A1. Location in patent: Page/Page column 4; 9
