39639-47-9
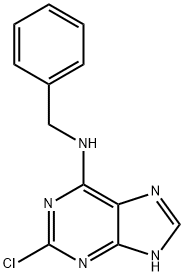 39639-47-9 结构式
39639-47-9 结构式
基本信息
N-苄基-2-氯-9H-嘌呤-6-胺
N-BENZYL-2-CHLORO-7H-PURIN-6-AMINE
N-Benzyl-2-chloro-9H-purin-6-amine
2-Chloro-N-(phenylmethyl)-1H-purin-6-amine
9H-Purin-6-amine, 2-chloro-N-(phenylmethyl)-
物理化学性质
制备方法
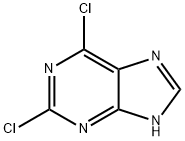
5451-40-1

100-46-9

39639-47-9
N-苄基-2-氯-9H-嘌呤-6-胺(4)的合成:将2,6-二氯嘌呤(110 mg,0.52 mmol)悬浮于正丁醇(3 mL)中,依次加入苄胺(57 mg,0.52 mmol)和三乙胺(72 mg,0.79 mmol)。将反应混合物于60℃搅拌加热15分钟。反应完成后,过滤收集沉淀,依次用水(20 mL)和甲醇(10 mL)洗涤,随后在空气中干燥过夜,得到灰白色固体产物4(130 mg,收率95%)。产物表征数据如下:熔点262℃;EI/MS m/z(相对丰度):259(19,M+),260(14),261(17%),106(100),91(77);1H NMR(400 MHz,DMSO-d6)δ 8.15(s,1H),7.25-7.34(m,5H),4.66(d,J = 6 Hz,2H);13C NMR(100 MHz,DMSO-d6)δ 155.0(s),153.1(s),150.7(s),140.2(d,1JC-H = 200 Hz),139.6(s),128.5(d,2JC-H = 158 Hz),127.5(d,3JC-H = 157 Hz),127.0(d,4JC-H = 158 Hz),118.1(s),43.4(t,2JC-H = 139 Hz)。
参考文献:
[1] Organic Process Research and Development, 2009, vol. 13, # 3, p. 641 - 644
[2] Synthetic Communications, 2010, vol. 40, # 12, p. 1856 - 1866
[3] Patent: WO2012/51296, 2012, A2. Location in patent: Page/Page column 40
[4] Archiv der Pharmazie, 1999, vol. 332, # 6, p. 187 - 190
[5] Nucleosides, Nucleotides and Nucleic Acids, 2011, vol. 30, # 7-8, p. 503 - 511