39824-26-5
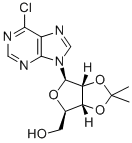 39824-26-5 结构式
39824-26-5 结构式
基本信息
6-氯-(2,3-0-异亚丙基)嘌呤核苷
6-氯-(2,3-O-异亚丙基)嘌呤核苷
6-氯嘌呤-9-(2,3-异丙基亚基-B-D-呋喃核糖苷)
6-CHLORO-9-BETA-D-(2,3-ISOPROPYLIDENE)RIBOFURANOSYLPURINE
6-CHLOROPURINE-9-(2,3-ISOPROPYLIDENE-B-D-RIBOFURANOSIDE)
6-CHLOROPURINE-9-(2,3-ISOPROPYLIDENE-BETA-D-RIBOFURANOSIDE)
6-Chloropurine-9-(2,3-isopropylidene)-b-d-ribfuraniside
6-CHLORO-9-[2,3-O-(ISOPROPYLIDENE)-β-D-RIBOFURONOSYL]-9H-PURINE
6-Chloro-9-b-D-(2,3-isopropylidene)ribofuranosylpurine
6-CHLORO-9-[2.3-0-(ISOPROPYLIDENE)-BETA-D-RIBOFURONOSYL]-9H-PURINE
6-Chloro-9-[2,3-o-(Isopropylid
6-Chloro-9-(2',3'-O-isopropylidene-b-D-ribofuranosyl)purine
6-chloro-9-[230-(isopropylidene)--D-ribofuronosyl]-9H-purine
6-CHLORO-9-[2,3-O-(ISOPROPYLIDENE)-SS-D-RIBOFURONOSYL]-9H-PURINE
6-CHLORO-9-[2'',3''-0-(ISOPROPYLIDENE)-SS-D-RIBOFURONOSYL]-9H-PURINE
6-Chloro-9--D-(2,3-isopropylidene)ribofuranosylpurine
6-Chloropurine-9-(2,3-isopropylidene--D-ribofuranoside)
物理化学性质
制备方法
2004-06-0

67-64-1

39824-26-5
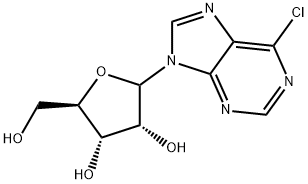
以(3R,4S,5R)-2-(6-氯-9H-嘌呤-9-基)-5-(羟甲基)四氢呋喃-3,4-二醇和丙酮为原料,合成(((3aR,4R,6R,6aR)-6-(6-氯-9H-嘌呤-9-基)-2,2-二甲基四氢呋喃并[3,4-d][1,3]二氧杂环戊烯-4-基)甲醇的一般步骤如下:向(3R,4S,5R)-2-(6-氯-9H-嘌呤-9-基)-5-(羟甲基)四氢呋喃-3,4-二醇(3.0g,10.5mmol)的无水丙酮(300mL)搅拌悬浮液中加入甲苯磺酸一水合物(19.8g,104mmol)。15分钟后,固体完全溶解。反应2小时后,将反应液缓慢倒入搅拌的0.5N NaHCO3水溶液(300mL)中。真空除去丙酮后,用二氯甲烷(DCM,100mL×5)萃取混合物。合并有机层,依次用水(100mL)和盐水(100mL)洗涤,经无水Na2SO4干燥,过滤并浓缩,得到目标产物(3.0g,产率87%,纯度>96%)为苍白色固体。产物经1H NMR(500MHz,CD3OD)和LCMS(m/z:327.1 [M+1]+)表征。
参考文献:
[1] Nucleosides and Nucleotides, 1996, vol. 15, # 1-3, p. 619 - 629
[2] Bioorganic and Medicinal Chemistry, 2008, vol. 16, # 7, p. 3848 - 3865
[3] ACS Medicinal Chemistry Letters, 2011, vol. 2, # 8, p. 577 - 582
[4] Patent: WO2012/82436, 2012, A2. Location in patent: Page/Page column 192-193
[5] Patent: WO2012/82436, 2012, A2. Location in patent: Page/Page column 256-257
