405-39-0
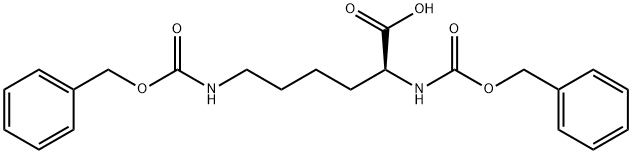 405-39-0 结构式
405-39-0 结构式
基本信息
N,N'-双苄氧羰基-L-赖氨酸
DI-N-CBZ-L-LYSINE
N-ALPHA,EPSILON-BIS-Z-L-LYSINE
N-ALPHA, N-EPSILON-DIBENZYLOXYCARBONYL-L-LYSINE
N-ALPHA-N-EPSILON-DICARBOBENZOXY-L-LYSINE
N-ALPHA-N-EPSILON-DI-CBZ-L-LYSINE
N-ALPHA,N-EPSILON-DI-Z-L-LYSINE
N,N'-DI-CBZ-L-LYSINE
Z-LYSINE(Z)-OH
Z-LYS(Z)-OH
Nα,Nε-Di-Z-L-lysine
di-N-CBZ-L-Lysine di-N-Carbobenzyloxy-L-lysine
N,N-di-cbz-L-lysine free acid
N2,N6-dibenzyloxycarbonyl-L-lysine
Cbz-Lys(Z)-OH
CBZ-LYS(CBZ)-OH
NA,NE-DIBENZYLOXYCARBONYL-L-LYSINE
Z-LYS(Z)
L-Lysine, N2,N6-bis[(phenylmethoxy)carbonyl]-
N-alpha-N-epsilon-Bis-benzyloxycarbonyl-L-lysine
物理化学性质
制备方法
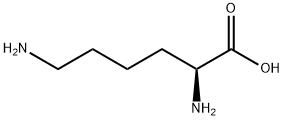
56-87-1

501-53-1

405-39-0
以L-赖氨酸和氯甲酸苄酯为原料合成(S)-2,6-双(((苄氧基)羰基)氨基)己酸的一般步骤如下,该合成方法参照Galbiati, B.; Ferrario, T.; Merli, V.的专利WP 0110851(2001)。在250 mL三颈烧瓶中依次加入水(20 mL)、1,4-二恶烷(20 mL)和L-赖氨酸(2.1 g,14.4 mmol),搅拌混合物直至完全溶解。用30% NaOH溶液调节pH至约10.5。在维持pH约10-11的条件下,缓慢加入氯甲酸苄酯(5.2 g,30.6 mmol),同时通过滴加30% NaOH溶液保持pH稳定。加料完毕后,将反应混合物在20℃下搅拌约1小时。随后,用37% HCl溶液调节pH至5。加入乙酸乙酯(30 mL),并用37% HCl溶液进一步调节pH至1。室温下搅拌混合物约30分钟后,分离有机层,水层用乙酸乙酯(20 mL)萃取。合并有机层,用盐水(30 mL)洗涤,经无水Na2SO4干燥。减压蒸发溶剂,得到淡黄色油状产物(6.0 g,收率99%)。最后,通过苯共沸蒸馏对产物进行预干燥处理。
参考文献:
[1] Patent: US2006/94673, 2006, A1. Location in patent: Page/Page column 4
[2] Patent: CN106565771, 2017, A. Location in patent: Paragraph 0037; 0038; 0039
[3] Patent: CN106565772, 2017, A. Location in patent: Paragraph 0038
[4] Helvetica Chimica Acta, 1958, vol. 41, p. 1867,1876
[5] Journal of Biological Chemistry, 1935, vol. 111, p. 245,251
