40601-76-1
中文名称
抗氧化剂 TH-1790
英文名称
Antioxidant 1790
CAS
40601-76-1
EINECS 编号
254-996-9
分子式
C42H57N3O6
MDL 编号
MFCD00192555
分子量
699.92
MOL 文件
40601-76-1.mol
更新日期
2026/02/27 13:56:42
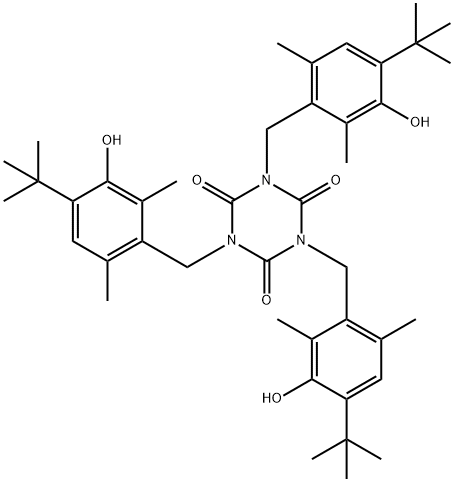 40601-76-1 结构式
40601-76-1 结构式
基本信息
中文别名
1,3,5-三(4-叔丁基-3-羟基-2,6-二甲基苄基)-1,3,5-三嗪-2,4,6-(1H,3H,5H)-三酮抗氧化剂 TH-1790
抗氧剂 1790
抗氧剂 XH-1790
1,3,5-三(4-叔丁基-3-羟基-2,6-二甲基苄基)-1,3,5-三嗪-2,4,6(1H,3H,5H)-三酮
1,3,5-三[[4-(1,1-二甲基乙基)-3-羟基-2,6-二甲基苯基]甲基]-1,3,5-三嗪-2,4,6-三酮
抗氧剂TH-1790
抗氧剂CY
防黄剂HN130
英文别名
1,3,5-tris(4-tert-butyl-3-hydroxy-2,6-dimethylbenzyl)-1,3,5-triazine-2,4,6-(1h,3h,5h)-trioneirganox 1790
Thanox 1790
TRIS(4-TERT-BUTYL-3-HYDROXY-2,6-DIMETHYLBENZYL) ISOCYANURATE
1,3,5-triazine-2,4,6(1h,3h,5h)-trione,1,3,5-tris[[4-(1,1-dimethylethyl)-3-hydr
1,3,5-Tris(4-t-butyl-3-hydroxy-2,6-dimethylbenzyl)-1,3,5-triazine-2,4,6(1H,3H,5H)-trione
3,5-Triazine-2,4,6(1H,3H,5H)-trione,1,3,5-tris[[4-(1,1-dimethylethyl)-3-hydroxy-2,6-dimethylphenyl]1
6-dimethylphenyl]methyl]-oxy-
tris(4-tert-butyl-3-hydroxy-2,6-dimethyl-benzyl)
Antioxidant 1790
1,3,5-tris[[4-tert-butyl-3-hydroxy-2,6-xylyl]methyl]-1,3,5-triazine-2,4,6(1H,3H,5H)-trione
1,3,5-Tris(4-tert-butyl-3-Hydroxy-2,6-Dimethyl Benzyl)-1,3,5-Triazine-2,4,6-(1H
1,3,5-Triazine-2,4,6(1H,3H,5H)-trione, 1,3,5-tris4-(1,1-dimethylethyl)-3-hydroxy-2,6-dimethylphenylmethyl-
1,3,5-TRIS(4-TERT-BUTYL-3-HYDROXY-2,6-DIMETHYLBENZYL)-1,3,.
1,3,5-TRlS(4-tert-butyl-3-hydroxy-2,6-dimethyl benzyl)–1,3,5-triazine-2,4,6-(1H,3H,5H)-trione
1,3,5-Tris-(4-tert-butyl-2,6-dimethyl-3-hydroxybenzyl)-isocyanurate
Irganox 3790(Ciba SC)Cyanox 1790(Cytec)
Yellow inhibitor HN130
1,3,5-Tris(3-hydroxy-4-tert-butyl-2,6-dimethylbenzyl)-1,3,5-triazine-2,4,6(1H,3H,5H)-trione
1,3,5-Tris(4-tert-butyl-3-hydroxy-2,6-dimethylbenzyl)-1,3,5-triazine-2,4,6-trione
所属类别
生物:小分子化合物物理化学性质
外观性状白色粉末。熔点158-162℃。
熔点163-165 °C(lit.)
沸点793.8±60.0 °C(Predicted)
密度1.172±0.06 g/cm3(Predicted)
蒸气压0Pa at 20℃
储存条件Sealed in dry,Room Temperature
溶解度可溶于乙腈(少许)、DMSO(少许)、甲醇(少许)
酸度系数(pKa)11.36±0.28(Predicted)
形态固体
颜色白色至灰白色
水溶解性20μg/L at 20℃
化妆品成分功效ANTIOXIDANT
InChIKeyXYXJKPCGSGVSBO-UHFFFAOYSA-N
SMILESN1(CC2=C(C)C=C(C(C)(C)C)C(O)=C2C)C(=O)N(CC2=C(C)C=C(C(C)(C)C)C(O)=C2C)C(=O)N(CC2=C(C)C=C(C(C)(C)C)C(O)=C2C)C1=O
LogP15.281 at 20℃
制备方法
方法1
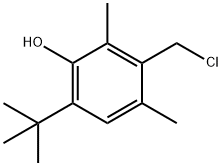
23500-79-0
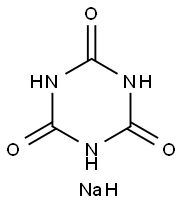
3047-33-4

40601-76-1
在装有搅拌器、回流冷凝器和温度计的500毫升四颈烧瓶中,加入氰尿酸三钠盐19克(0.1摩尔)、2-叔丁基-3-氯甲基-4,6-二甲基苯酚71克(0.33摩尔)、N,N-二甲基甲酰胺200毫升和相转移催化剂1克。在连续氮气保护下,将反应混合物加热至120°C并搅拌反应14小时。反应完成后,将混合物冷却至50-60°C并过滤。滤液在0.005-0.15MPa压力下蒸馏除去N,N-二甲基甲酰胺。向残余物中加入200毫升甲苯和100毫升水,搅拌至所有物料完全溶解后,静置分层,分离水相。有机相蒸馏除去剩余的水和约160毫升甲苯。缓慢加入150毫升甲醇,搅拌并回流至物料完全溶解。加入5克活性炭进行脱色,热过滤后,冷却结晶,过滤,洗涤,得到干燥的1,3,5-三(4-(叔丁基)-3-羟基-2,6-二甲基苄基)-1,3,5-三嗪烷-2,4,6-三酮54.6克,熔点157-160°C,纯度98.3%。产物通过红外光谱进行表征。
参考文献:
[1] Patent: CN103420932, 2016, B. Location in patent: Paragraph 0024; 0025; 0026
[2] Patent: CN106699678, 2017, A. Location in patent: Paragraph 0052; 0053
抗氧化剂 TH-1790价格(试剂级)
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | T2974 | 抗氧化剂 TH-1790 1,3,5-Tris[4-(tert-butyl)-3-hydroxy-2,6-dimethylbenzyl]-1,3,5-triazinane-2,4,6-trione | 40601-76-1 | 25G | 1490元 |
| 2025/12/22 | T2974 | 抗氧化剂 TH-1790 1,3,5-Tris[4-(tert-butyl)-3-hydroxy-2,6-dimethylbenzyl]-1,3,5-triazinane-2,4,6-trione | 40601-76-1 | 100G | 5650元 |
| 2025/05/22 | XW406017611 | 异氰脲酸三(4-叔丁基-3-羟基-2,6-二甲苯基)酯 | 40601-76-1 | 25G | 164元 |
