408492-27-3
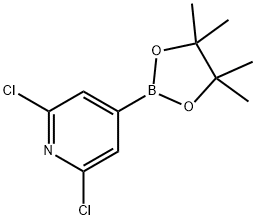 408492-27-3 结构式
408492-27-3 结构式
基本信息
2,6-二氯吡啶-4-硼酸频哪醇酯
2,6-二氯吡啶-4-硼酸频呢醇酯
2,6-二氯-4-(4,4,5,5-四甲基-1,3,2-二氧杂环戊硼烷-2-基)吡啶
2,6-Dichloropyridine-4-boronic acid,pinacol ester
2,6-Dichloropyridinyl-4-boronic acid pinacol ester
(2,6-DICHLOROPYRIDIN-4-YL)BORONIC ACID PINACOL ESTER
2,6-Dichloro-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)
2,6-DICHLORO-4-(TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)PYRIDINE
2-(2,6-Dichloro-4-pyridyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
2,6-DICHLORO-4-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)PYRIDINE
Pyridine, 2,6-dichloro-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-
2,6-DICHLORO-4-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)PYRIDINE ISO 9001:2015 REACH
物理化学性质
制备方法
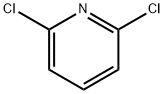
2402-78-0

73183-34-3

408492-27-3
以2,6-二氯吡啶和联硼酸频那醇酯为原料合成2,6-二氯吡啶-4-硼酸频哪醇酯的一般步骤:在氮气保护下,将2,6-二氯吡啶(3g,20.3mmol)、4,4,5,5-四甲基-1,3,2-二氧杂硼杂环戊烷-2-基(5.92g,23.3mmol)、1,10-菲咯啉(145mg,0.81mmol)和氯代双(1,5-环辛二烯)铱(I)二聚体(267mg,0.30mmol)溶于1,2-二氯乙烷(20mL)中。通入氮气5分钟后,将反应混合物在100℃下加热1小时。反应完成后,将冷却的混合物倒入乙醚(150mL)和4M氢氧化钠水溶液(200mL)的混合液中,分离有机相和水相。水相用冰浴冷却后,用5M盐酸水溶液酸化,过滤产生的沉淀,用水洗涤并干燥,得到2,6-二氯吡啶-4-硼酸频哪醇酯(4.9g,17.9mmol,收率88%)。LCMS(方法A):保留时间0.74分钟;m/z 192,194(作为硼酸离子化的MH+)。1H NMR(400MHz,DMSO-d6)δ 1.28(12H,s),7.57(2H,s)。
参考文献:
[1] Organic Letters, 2009, vol. 11, # 16, p. 3586 - 3589
[2] Journal of the American Chemical Society, 2015, vol. 137, # 25, p. 8058 - 8061
[3] Organic and Biomolecular Chemistry, 2014, vol. 12, # 37, p. 7318 - 7327
[4] Patent: WO2011/110575, 2011, A1. Location in patent: Page/Page column 73
[5] Journal of the American Chemical Society, 2018, vol. 140, # 49, p. 17197 - 17202
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | D5574 | 2,6-二氯-4-(4,4,5,5-四甲基-1,3,2-二氧杂环戊硼烷-2-基)吡啶 2,6-Dichloro-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine | 408492-27-3 | 1g | 180元 |
| 2025/12/22 | XW0240849227304 | 2,6-二氯吡啶-4-硼酸频哪醇酯 | 408492-27-3 | 10G | 593元 |
| 2025/12/22 | XW0240849227303 | 2,6-二氯吡啶-4-硼酸频哪醇酯 | 408492-27-3 | 5G | 297元 |
