41175-50-2
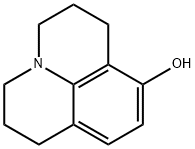 41175-50-2 结构式
41175-50-2 结构式
基本信息
2,3,6,7-TETRAHYDRO-1H,5H-PYRIDO[3,2,1-IJ]QUINOLIN-8-OL
8-HYDROXY-2,3,6,7-TETRAHYDRO-1H,5H-BENZO[IJ]QUINOLIZINE
8-HYDROXYJULOLIDINE
8-HYDROXYJULOLIDINE HYDROCHLORIDE
AKOS BBS-00000819
TIMTEC-BB SBB006615
,3,6,7-Tetrahydro-1H,5H-benzo(ij)quinolizin-8-ol
,3,6,7-Tetrahydro-1H,5H-pyrido[3,2,1-ij]quinolin-8-ol
5h-benzo[ij]quinolizin-8-ol,2,3,6,7-tetrahydro-1
8-HydroxyIulolidineHCl
1H,5H-Benzoijquinolizin-8-ol, 2,3,6,7-tetrahydro-
物理化学性质
hematology
histology
安全数据
Skin Irrit. 2
STOT SE 3
制备方法
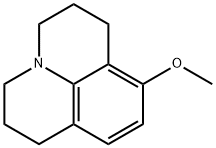
63468-83-7

41175-50-2
以8-甲氧基久洛利定为原料合成1,2,3,5,6,7-六氢吡啶并[3,2,1-ij]喹啉-8-醇的一般步骤如下:将8-甲氧基久洛利定(10g,50mmol)溶解于由50mL 47%氢碘酸、80mL浓盐酸和200mL水组成的混合溶液中。将反应混合物加热回流,并通过薄层色谱(TLC)监测反应进度。反应15小时后,向混合物中追加50mL浓盐酸。总反应时间为60小时。反应完成后,将溶液置于冰浴中冷却,首先用50%氢氧化钠溶液中和至pH6,随后加入磷酸盐缓冲液(由6.9g NaH2PO4·H2O和1.4g Na2HPO4溶于100mL水配制而成)。用二氯甲烷萃取产物,有机相用盐水洗涤后,以无水硫酸钠干燥,减压浓缩去除溶剂。将所得粗产物重新溶解于二氯甲烷中,用10%氢氧化钠溶液反复萃取直至水相无色。随后,酸化有机相,干燥后再次萃取,按上述方法去除溶剂,最终得到产物1,2,3,5,6,7-六氢吡啶并[3,2,1-ij]喹啉-8-醇(6.24g),产率为67%,熔点为126-130℃。
参考文献:
[1] Journal of Organic Chemistry, 1987, vol. 52, # 8, p. 1465 - 1468
[2] Journal of Fluorescence, 2015, vol. 25, # 6, p. 1615 - 1628
[3] Chemical Communications, 2011, vol. 47, # 47, p. 12691 - 12693
[4] Chemistry - A European Journal, 2014, vol. 20, # 12, p. 3510 - 3519
[5] Patent: US2018/280543, 2018, A1. Location in patent: Paragraph 0041; 0098
