41332-24-5
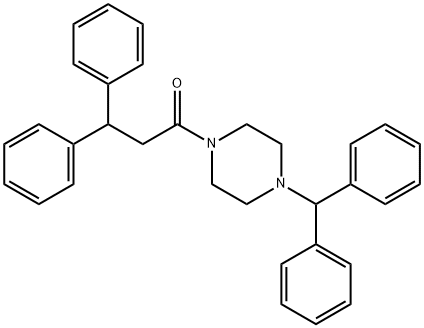 41332-24-5 结构式
41332-24-5 结构式
基本信息
1-【4-(二苯甲基)-1-哌嗪基】-3,3-二苯基-1-丙酮
1-[4-(二苯基甲基)-1-哌嗪]-3,3-二苯基-1-丙酮
39-1B4
CS-2371
NP 118809
NP118809 (39-1B4
39-1B4
NP-118809
NP 118809
NP118809
1-(DiphenylMethyl)-4-(1-oxo-3,3-diphenylpropyl)piperazine
Piperazine, 1-(diphenylmethyl)-4-(1-oxo-3,3-diphenylpropyl)-
1-[4-(DiphenylMethyl)-1-piperazinyl]-3,3-diphenyl-1-propanone
物理化学性质
制备方法
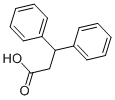
606-83-7
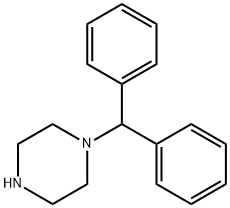
841-77-0

41332-24-5
1-(Diphenylmethyl)-4-(1-oxo-3,3-diphenylpropyl)piperazine的合成步骤:向1-(Diphenylmethyl)piperazine(2.08 mmol)的无水二氯甲烷(40 mL)溶液中,在氮气保护下加入3,3-diphenylpropionic acid(0.472 g,2.08 mmol)。随后向反应混合物中加入EDC(0.797 g,4.16 mmol)和催化量的DMAP,反应混合物在室温下氮气氛围中搅拌过夜。反应完成后,将混合物减压浓缩。将残余物溶解于乙酸乙酯与水的混合溶剂(10:1,150 mL)中。有机相依次用水(30 mL,2次)和10% NaOH溶液(30 mL)洗涤,用无水硫酸镁干燥后,减压蒸发除去溶剂。所得粗产物通过柱色谱法纯化,洗脱剂为石油醚与乙酸乙酯的混合溶剂(3:1),最终得到目标产物1-(diphenylmethyl)-4-(1-oxo-3,3-diphenylpropyl)piperazine,产率为78%。
参考文献:
[1] Patent: US2004/259866, 2004, A1
[2] Patent: US6951862, 2005, B2
[3] Bioorganic and Medicinal Chemistry Letters, 2009, vol. 19, # 22, p. 6467 - 6472
[4] Bioorganic and Medicinal Chemistry Letters, 2010, vol. 20, # 4, p. 1378 - 1383
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | HY-14462 | 1-【4-(二苯甲基)-1-哌嗪基】-3,3-二苯基-1-丙酮 NP118809 | 41332-24-5 | 100mg | 744元 |
| 2025/12/22 | HY-14462 | 1-【4-(二苯甲基)-1-哌嗪基】-3,3-二苯基-1-丙酮 NP118809 | 41332-24-5 | 10mM * 1mLin DMSO | 818元 |
| 2025/12/22 | HY-14462 | 1-【4-(二苯甲基)-1-哌嗪基】-3,3-二苯基-1-丙酮 NP118809 | 41332-24-5 | 200mg | 930元 |
常见问题列表
|
N-Type Ca 2+ Channel 0.11 μM (IC 50 ) |
L-type calcium channel 12.2 μM (IC 50 ) |
NP118809 is a potent N-type calcium channel blocker, with an IC 50 of 0.11 μM; also inhibits L-type calcium channel with an IC 50 of 12.2 μM. NP118809 inhibits the hERG potassium channel in HEK cells, with an IC 50 of 7.4 μM.
NP118809 (25 mg/kg, i.p.) shows significant analgesic activity in the phase IIA portions of the rat formalin model. NP118809 (30 mg/kg, p.o.) results in 80.3% inhibition of mechanical allodynia and 96.3% inhibition of thermal hyperalgesia in the rat spinal nerve ligation model.
