43229-65-8
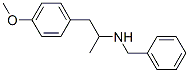 43229-65-8 结构式
43229-65-8 结构式
基本信息
对-甲氧苯基-2-苄胺基丙烷
4-苯基苄胺
N-Benzyl-4-methoxy-alpha-methylphenethylamine
P-METHOXYPHENYL-2-BENZYLAMINOPROPANE
1-(4-METHOXYPHENYL)-2-BENZYL AMINO PROPANE ,98%MIN.
N-[BENZYL-N-( 1-METHYL-2-P-METHOXYPHENYLETHYL)]AMINE
4-Methoxy-α-methyl-N-(phenylmethyl)benzeneethanamine
N-Benzyl-N-(4-methoxy-α-methylphenethyl)amine
物理化学性质
制备方法
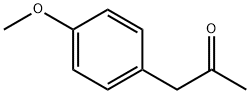
122-84-9

100-46-9

43229-65-8
通用方法:将1-(4-甲氧基苯基)丙-2-酮(8.22 g, 50.0 mmol)和苄胺(5.35 g, 50.0 mmol)溶解于甲醇中,加入5% Pt/C催化剂(0.83 g)。将反应混合物在60°C下搅拌,并进行氢化反应24小时。反应完成后,通过过滤移除催化剂,并通过旋转蒸发除去溶剂,得到中间体N-苄基-1-(4-甲氧基苯基)丙-2-胺,产率为95%。产物经1H NMR (400 MHz, CDCl3)表征:δ 7.25 (ddd, J = 16.5, 11.1, 6.8 Hz, 5H), 7.07 (d, J = 8.5 Hz, 2H), 6.83 (d, J = 8.5 Hz, 2H), 3.86 (d, J = 13.3 Hz, 1H), 3.79 (s, 3H), 3.73 (d, J = 13.3 Hz, 1H), 2.90 (dd, J = 13.0, 6.5 Hz, 1H), 2.71 (dd, J = 13.5, 7.1 Hz, 1H), 2.61 (dd, J = 13.5, 6.4 Hz, 1H), 1.10 (d, J = 6.2 Hz, 3H)。LC/MS (ESI m/z): 256.4 (M + H)+。
参考文献:
[1] Tetrahedron Letters, 1997, vol. 38, # 7, p. 1125 - 1128
[2] Organic Process Research and Development, 1998, vol. 2, # 2, p. 96 - 99
[3] Journal of Medicinal Chemistry, 2007, vol. 50, # 12, p. 2903 - 2915
[4] Bioorganic and Medicinal Chemistry Letters, 2014, vol. 24, # 1, p. 249 - 253
[5] Patent: US2011/313199, 2011, A1. Location in patent: Page/Page column 14

