4541-15-5
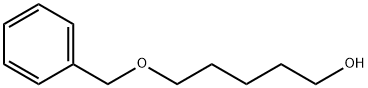 4541-15-5 结构式
4541-15-5 结构式
基本信息
5-苄氧基-1-戊醇
ENZYLOXY-1-PENTANOL
5-BENZYLOXY-1-PENTANOL
5-(benzyloxy)pentan-1-ol
5-(Benzyloxy)-1-pentanenol
1-pentanol, 5-(benzyloxy)-
5-Benzyloxy-1-pentanol 95%
5-(phenylmethoxy)pentan-1-ol
1-Pentanol,5-(phenylMethoxy)-
物理化学性质
制备方法

111-29-5

100-39-0

4541-15-5
向搅拌的60%氢化钠(NaH)在矿物油中的分散体(2.30g,57.69mmol)悬浮液中,于0°C下缓慢滴加1,5-戊二醇(5.0g,48.07mmol)的无水N,N-二甲基甲酰胺(DMF,150mL)溶液,历时15分钟。滴加完毕后,将反应混合物在室温下搅拌1小时。随后,在15分钟内缓慢加入苄基溴(5.75mL,48.07mmol),并在相同温度下继续搅拌2小时。反应完成后,用冰冷的水淬灭反应混合物。粗产物用乙酸乙酯(2×30mL)萃取,合并有机层,用饱和食盐水洗涤(3次),无水硫酸钠(Na2SO4)干燥,减压浓缩。残留物通过硅胶柱层析纯化,以石油醚-乙酸乙酯(7:3,v/v)为洗脱剂,得到目标产物5-苄氧基-1-戊醇。产率:89%(7.87g),为无色油状液体。红外光谱(CHCl3,cm-1):υmax 700, 714, 1028, 1072, 1116, 1177, 1278, 1316, 1388, 1453, 2865, 2938, 3064, 3332;1H NMR(200MHz,CDCl3):δ 1.47-1.70(m,6H),3.47(t,J = 6.3Hz,2H),3.62(t,J = 6.1Hz,2H),4.49(s,2H),7.31(s,5H);13C NMR(50MHz,CDCl3):δ 22.3, 29.3, 32.3, 62.2, 70.2, 72.8, 127.5, 128.2, 138.3;高分辨质谱(ESI):计算值 [C12H18O2 + H]+ (M + H) 195.1385,实测值195.1390。
参考文献:
[1] Journal of the American Chemical Society, 2007, vol. 129, # 5, p. 1465 - 1469
[2] Organic Letters, 2016, vol. 18, # 7, p. 1622 - 1625
[3] Journal of the American Chemical Society, 2015, vol. 137, # 1, p. 420 - 424
[4] Tetrahedron Letters, 2016, vol. 57, # 1, p. 25 - 28
[5] Helvetica Chimica Acta, 2015, vol. 98, # 10, p. 1395 - 1402
