51834-97-0
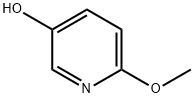 51834-97-0 结构式
51834-97-0 结构式
基本信息
5-羟基-2-甲氧基嘧啶
5-羟基-2-甲氧基吡啶
5-羟基-2-M乙氧基吡啶
3-Pyridinol, 6-methoxy-
5-HYDROXY-2-METHOXYPYRIDIN
6-METHOXY-HYDROXY-PYRIDINE
5-hydroxy-2-methoxypridine
3-Pyridinol,6-methoxy-(9CI)
5-HYDROXY-2-METHOXYPYRIDINE
5-Hydroxy-2-methoxylpyridine
5-HYDROXY-2-METHOXYPYRIDINE ISO 9001:2015 REACH
2-Methoxyl-5-hydroxypyridine,6-Methoxypyridin-3-ol
物理化学性质
制备方法
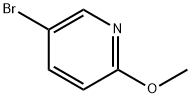
13472-85-0

51834-97-0
步骤2. 5-羟基-2-甲氧基吡啶的合成: 在-78℃条件下,向搅拌中的5-溴-2-甲氧基吡啶(8.9g,47.9mmol)的四氢呋喃(THF,175mL)溶液中缓慢滴加正丁基锂溶液(2.5M己烷溶液,28.7mL,71.8mmol)。反应混合物在-78℃下持续搅拌45分钟。随后,通过注射器加入硼酸三甲酯(7.06mL,62.2mmol),并将混合物在相同温度下继续搅拌2小时。将反应混合物升温至0℃,并加入3N NaOH溶液(25mL,71.77mmol)和30%过氧化氢溶液(约50mL)的混合液进行处理。反应混合物逐渐变为黄色并略显混浊,随后升温至室温,保持30分钟后加热至回流温度,维持1小时。反应完成后,将混合物冷却至室温。水层用1N HCl溶液中和,随后用乙醚(Et2O,2×100mL)萃取。合并有机层,用无水硫酸钠(Na2SO4)干燥,减压浓缩,得到黄色粘稠油状产物(3.5g,收率60%)。
参考文献:
[1] Medicinal Chemistry Research, 2013, vol. 22, # 4, p. 1825 - 1836
[2] Heterocycles, 2002, vol. 57, # 1, p. 55 - 71
[3] Patent: EP1449834, 2004, A2. Location in patent: Page 27
[4] Patent: EP1042305, 2005, B1. Location in patent: Page/Page column 33
[5] Patent: US2003/207914, 2003, A1. Location in patent: Page/Page column 12
