51839-15-7
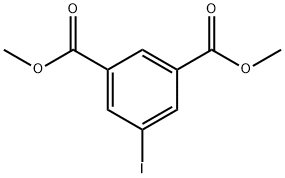 51839-15-7 结构式
51839-15-7 结构式
物理化学性质
安全数据
制备方法
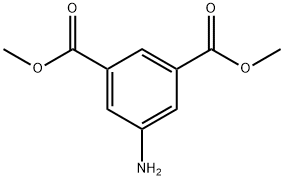
99-27-4

51839-15-7
在氩气保护下,将5-氨基间苯二甲酸二甲酯(1)(6.27 g,30.0 mmol)加入至预先用冰浴冷却的39 mL 2M盐酸中。反应混合物在0℃或更低温度下搅拌数分钟后,缓慢滴加21.6 mL预先冰浴冷却的亚硝酸钠水溶液(2.52 g,36.5 mmol,1.2当量)。随后,加入30 mL二氯甲烷,待反应混合物升至室温后继续搅拌5小时。反应完成后,在冰浴冷却条件下缓慢滴加70 mL碘化钾水溶液(7.47 g,45.0 mmol,1.5当量),并将混合物在室温下搅拌12小时。反应结束后,用乙酸乙酯萃取水层,有机层用饱和食盐水洗涤,无水硫酸镁干燥,经硅胶柱色谱纯化(硅胶,己烷为洗脱剂),得到目标产物5-碘间苯二甲酸二甲酯(2)(5.285 g,16.5 mmol,收率55%)。产物表征数据如下:1H-NMR (400 MHz, CDCl3) δ: 8.62 (t, 1H, J = 1.4 Hz), 8.53 (s, 2H, J = 1.4 Hz), 3.94 (s, 6H); 13C-NMR (100 MHz, CDCl3) δ: 165.2 (2C), 142.9 (2C), 132.6 (2C), 130.2, 93.8, 53.1 (2C); MS (EI) m/z: 320 (M+); HRMS (EI) calculated for C10H9IO4: 319.9546, found: 319.9553; Elemental analysis calculated for C10H9O4: C, 37.52; H, 2.83; found: C, 37.44; H, 2.86.
参考文献:
[1] Inorganic Chemistry, 2015, vol. 54, # 9, p. 4377 - 4381
[2] Journal of the American Chemical Society, 2012, vol. 134, # 49, p. 20110 - 20116
[3] Organic and Biomolecular Chemistry, 2011, vol. 9, # 18, p. 6284 - 6292
[4] Chemical Communications, 2011, vol. 47, # 9, p. 2691 - 2693
[5] Patent: US2018/94017, 2018, A1. Location in patent: Paragraph 0061; 0064; 0065
