52133-67-2
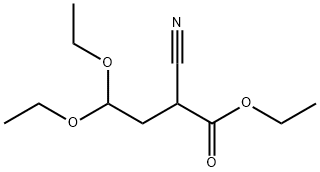 52133-67-2 结构式
52133-67-2 结构式
基本信息
ETHYL 2,2-DIETHOXYETHYLCYANOACETATE
ETHYL 2-CYANO-4,4-DIETHOXYBUTANOATE
ETHYL 2-CYANO-4,4-DIETHOXYBUTYRATE
ETHYL-2,2-DIETHOXYTHYLCYANOACETATE
Ethyl-2-Cyano-4,4-Diethoxybutanoide
安全数据
制备方法

105-56-6
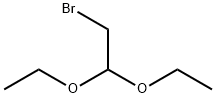
2032-35-1

52133-67-2
方法AI:2-氰基-4,4-二乙氧基丁酸乙酯(xlii-a)的合成:在干燥的反应瓶中,依次加入2-氰基乙酸乙酯(11.4g,101mmol,5.0当量)、碳酸钾(2.8g,20mmol,1.0当量)和碘化钠(200mg,1.3mmol,0.06当量)。随后,向混合物中缓慢加入2-溴-1,1-二乙氧基乙烷(4g,20mmol,1.0当量)。参照J.Chem.Soc.,1960,131-138的方法,将反应混合物在145℃下回流4小时。反应完成后,冷却至室温,通过硅胶柱色谱法纯化产物,洗脱剂为石油醚/乙酸乙酯(梯度洗脱:80:1→40:1→40:1),得到3.57g目标化合物xii-a,为无色油状物,收率78%。1H NMR(400MHz,CDCl3):δ4.70(t,J=5.6Hz,1H),4.26(q,J=7.2Hz,2H),3.78-3.64(m,3H),3.62-3.45(m,2H),2.35-2.14(m,2H),1.34(q,J=7.2Hz,3H),1.25-1.16(m,6H)。
参考文献:
[1] Patent: US2015/307477, 2015, A1. Location in patent: Paragraph 1563
[2] Patent: JP6121658, 2017, B2. Location in patent: Paragraph 1270; 1271
[3] Archiv der Pharmazie, 2018, vol. 351, # 8,
[4] Bioorganic and Medicinal Chemistry Letters, 2012, vol. 22, # 12, p. 4064 - 4067
[5] Organic Letters, 2017, vol. 19, # 9, p. 2214 - 2217
