52498-32-5
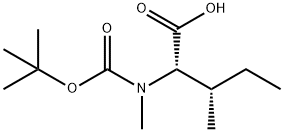 52498-32-5 结构式
52498-32-5 结构式
基本信息
BOC-L-MELLE-OH
BOC-MEILE-OH
BOC-MELLE-OH
BOC-N-ALPHA-METHYL-L-ISOLEUCINE
BOC-N-ME-ILE-OH
BOC-N-ME-ISOLEUCINE
BOC-N-METHYL-L-ILE-OH
BOC-N-METHYL-L-ISOLEUCINE
N-ALPHA-T-BOC-N-ALPHA-METHYL-L-ISOLEUCINE
N-ALPHA-T-BUTOXYCARBONYL-N-ALPHA-METHYL-L-ISOLEUCINE
N-ALPHA-T-BUTYLOXYCARBONYL-N-ALPHA-METHYL-L-ISOLEUCINE
N-ALPHA-TERT-BUTYLOXYCARBONYL-N-ALPHA-METHYL-L-ISOLEUCINE
N-ALPHA-TERT-BUTYLOXYCABONYL-N-ALPHA-METHYL-L-ISOLEUCINE
N-alpha-t-Butyloxycarbonyl-N-alpha-methy-L-isoleucine
NALPHA-tert-Butoxycarbonyl-N-methyl-L-isoleucine
N-tert-Butyloxycarbonyl-N-methyl-L-isoleucine
物理化学性质
制备方法
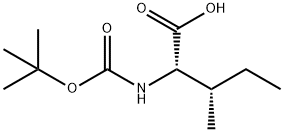
13139-16-7

74-88-4
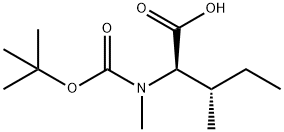
53462-50-3
将N-(叔丁氧基羰基)-L-异亮氨酸(1.24 g,5.4 mmol,1.0当量)溶于35 mL四氢呋喃(THF)中,冷却至0℃,缓慢加入氢化钠(60%分散于矿物油中;0.64 g,16.1 mmol,3.0当量)。随后加入碘甲烷(6.1 g,42 mmol,8.0当量),反应混合物在室温下搅拌24小时。反应完成后,加入乙醚,有机层用去离子水洗涤两次。合并的水层用柠檬酸调节pH至3,然后用乙酸乙酯(EtOAc)萃取。合并的有机相依次用硫代硫酸钠(Na2S2O3)溶液和饱和食盐水洗涤,无水硫酸钠(Na2SO4)干燥。减压除去溶剂,得到(2S,3S)-2-((叔丁氧羰基)(甲基)氨基)-3-甲基戊酸,为无色油状物(1.18 g,收率90%)。产物经红外光谱(IR)、核磁共振氢谱(1H NMR)、核磁共振碳谱(13C NMR)和高分辨质谱(HRMS)表征,确认结构正确。比旋光度[α]20D为+2.6(c 1.8,乙酸)。
参考文献:
[1] Acta Chimica Slovenica, 2016, vol. 63, # 2, p. 344 - 350
[2] Tetrahedron, 2018, vol. 74, # 38, p. 5138 - 5142
[3] Tetrahedron Letters, 2018, vol. 59, # 30, p. 2938 - 2940
[4] Journal of Natural Products, 2014, vol. 77, # 8, p. 1871 - 1880
[5] Tetrahedron, 1991, vol. 47, # 29, p. 5453 - 5462
