53449-14-2
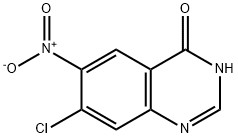 53449-14-2 结构式
53449-14-2 结构式
基本信息
6-硝基-7-氯喹唑啉-4-酮
7-氯-6-硝基喹唑啉-4-酮
7-氯-6-硝基-4-羟基喹唑啉
7-氯-4-羟基-6-硝基喹唑啉
7-氯-6-硝基喹唑啉-4(3H)-酮
7-氯-6-硝基喹唑啉-4-酮 25G
4(3H)-喹唑啉酮, 7-氯-6-硝基-
7-Chloro-6-nitro-4-quinazolinol
7-Chloro-6-nitroquinazolin-4(3H)
7-Chloro-6-nitroquinazolin-4-one
7-Chloro-6-nitro-4-quinazolinone
7-CHLORO 6-NITRO QUINAZOLINE 4-OL
7-Chloro-6-nitro-4(3H)quinazolinone
6-Nitro-7-Chloro-4-HydroxyQuizoline
7-Chloro-6-nitro-3H-quinazolin-4-one
7-Chloro-6-nitroquinazolin-4(1H)-one
物理化学性质
制备方法
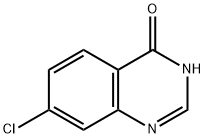
31374-18-2

53449-14-2
以7-氯-4(3H)-喹唑啉酮为原料合成7-氯-4-羟基-6-硝基喹唑啉的一般步骤:向100 mL圆底烧瓶中加入浓硫酸(10 mL)和浓硝酸(5 mL),随后加入7-氯-4(3H)-喹唑啉酮(10 g,55.4 mmol)。将反应混合物在0℃下搅拌,然后缓慢加热至90℃,并在该温度下持续搅拌3小时。反应完成后,将混合物冷却至室温,并小心倒入冰水中。通过过滤收集沉淀,用水充分洗涤,然后干燥。粗产物通过从乙酸中重结晶纯化,得到7-氯-6-硝基喹唑啉-4(3H)-酮(6c),为浅黄色固体(9 g,收率72%)。产物的熔点为315-316℃;1H NMR(DMSO-d6,ppm):δ 12.79(宽峰,1H),8.67(单峰,1H),8.31(单峰,1H),8.01(单峰,1H)。
参考文献:
[1] Medicinal Chemistry Research, 2013, vol. 22, # 9, p. 4096 - 4109
[2] European Journal of Medicinal Chemistry, 2018, vol. 147, p. 227 - 237
[3] Patent: CN108484574, 2018, A. Location in patent: Paragraph 0133; 0134; 0135
[4] Patent: CN103382182, 2016, B. Location in patent: Paragraph 0272; 0273; 0276; 0277
[5] Patent: WO2008/33747, 2008, A2. Location in patent: Page/Page column 129
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | C2332 | 7-氯-6-硝基-4-羟基喹唑啉 7-Chloro-6-nitro-4-hydroxyquinazoline | 53449-14-2 | 5G | 320元 |
| 2025/12/22 | C2332 | 7-氯-6-硝基-4-羟基喹唑啉 7-Chloro-6-nitro-4-hydroxyquinazoline | 53449-14-2 | 25G | 1175元 |
| 2025/05/22 | XW534491422 | 7-氯-6-硝基-4-羟基喹唑啉 7-chloro-6-nitro-4(3h)quinazolinone;7-chloro-6-nitro-4-hydroxyquinazoline;7-chloro-4-hydroxy-6-nitroquinazoline | 53449-14-2 | 25G | 241元 |
