54346-19-9
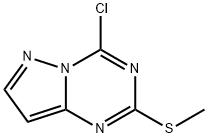 54346-19-9 结构式
54346-19-9 结构式
基本信息
pyrazolo[1,5-a][1,3,5]triazine
4-chloro-2-(Methylthio)pyrazolo[1
4-CHLORO-2-METHYLTHIOPYRAZOLO[1,5-A]1,3,5-TRIAZINE
Pyrazolo[1,5-a]-1,3,5-triazine, 4-chloro-2-(methylthio)-
4-Chloro-2-methylsulfanyl-pyrazolo[1,5-a][1,3,5]triazine
4-chloro-2-(methylthio)-1,2-dihydropyrazolo[1,5-a][1,3,5]triazine
物理化学性质
制备方法
![2 - (甲硫基)吡唑并[1,5-A][1,3,5]三嗪-4 - 醇](/CAS/GIF/54346-18-8.gif)
54346-18-8
![4-氯-2-(甲硫基)吡唑并[1,5-A][1,3,5]三嗪](/CAS/GIF/54346-19-9.gif)
54346-19-9
以2-(甲硫基)吡唑并[1,5-α][1,3,5]三嗪-4(3H)-酮为原料合成4-氯-2-(甲硫基)吡唑并[1,5-a][1,3,5]三嗪的一般步骤如下:在室温下,向搅拌中的2-(甲硫基)吡唑并[1,5-α][1,3,5]三嗪-4(3H)-酮(2.0g,11mmol)和三氯氧磷(20mL)的悬浮液中加入N,N-二乙基苯胺(0.7mL)。将反应混合物加热至回流温度。反应1.5小时后,将混合物冷却至室温并减压浓缩。将残余物溶解于甲苯中,有机层用盐水洗涤后再次减压浓缩,得到4-氯-2-(甲硫基)吡唑并[1,5-a][1,3,5]三嗪(2.0g,收率91%)。TLC Rf = 0.57(乙酸乙酯/己烷,1/3);1H NMR(300MHz,CDCl3)δ 8.15(d,J = 2.2Hz,1H),6.50(d,J = 2.2Hz,1H),2.60(s,3H)。
参考文献:
[1] Bioorganic and Medicinal Chemistry, 2011, vol. 19, # 20, p. 5955 - 5966
[2] Patent: US2007/78136, 2007, A1. Location in patent: Page/Page column 50
[3] Patent: WO2011/31979, 2011, A1. Location in patent: Page/Page column 147
[4] Patent: US9303033, 2016, B2. Location in patent: Page/Page column 285; 286
[5] Patent: JP5802676, 2015, B2. Location in patent: Paragraph 0552