54535-22-7
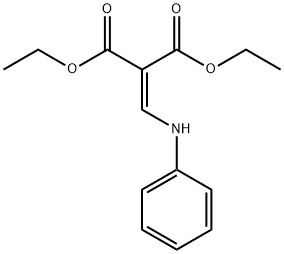 54535-22-7 结构式
54535-22-7 结构式
基本信息
2-苯基氨基亚甲基-丙二酸二乙酯
diethyl 2-(anilinomethylene)propanedioate
ANILINOMETHYLENEMALONIC ACID DIETHYL ESTER
diethyl 2-((phenylamino)methylene)malonate
2-PHENYLAMINOMETHYLENE-MALONIC ACIDDIETHYL ESTER
Propanedioic acid, 2-[(phenylaMino)Methylene]-, 1,3-diethyl ester
Propanedioic acid,2-[(phenylamino)methylene]-, 1,3-diethyl ester (Related Reference)
物理化学性质
制备方法

62-53-3
87-13-8

54535-22-7
将苯胺(2.733 mL,29.99 mmol)与乙氧基甲叉丙二酸二乙酯(6.063 mL,30.00 mmol)混合,在120-130℃下搅拌反应16.5小时。通过薄层色谱(TLC,展开剂为乙酸乙酯:环己烷=1:1)监测反应进程,确认反应完全(原料点消失,出现一个新的UV活性点,Rf值为0.84)。反应完成后,将反应液冷却至室温,得到中间体2-苯基氨基亚甲基-丙二酸二乙酯(35),为深黄色结晶固体(7.899 g,定量收率)。该化合物的熔点为36-37℃。高分辨质谱(HRMS-EI)分析显示:[M]+实测值为263.11531,与理论值C14H17NO4(263.11521)相符。红外光谱(薄膜法,cm-1):3265, 3184(弱,NH伸缩振动),3050(弱,芳环C-H伸缩振动),2981, 2936, 2904, 2871(中强,烷基C-H伸缩振动),1717(强,分子内氢键C=O与C=C共轭),1691(强,C=C-NH),1655(强,C=N-),1623(强,芳环共轭C=C),1255(强,C-N伸缩振动)。核磁共振氢谱(500 MHz, CD3CN):δ 1.31(3H,三重峰,J=7.1 Hz,CH3),1.32(3H,三重峰,J=7.2 Hz,CH3),4.19(2H,四重峰,J=7.2 Hz,CH2),4.25(2H,四重峰,J=7.1 Hz,CH2),7.16(1H,三重峰,J=7.4 Hz,para-ArH),7.20(2H,双三重峰,J=7.6 Hz,ortho-ArH),7.38(2H,多重峰,J=7.4 Hz,meta-ArH),8.48(1H,双峰,J=13.8 Hz,CH-NH),10.81(1H,双峰,J=13.6 Hz,CH-NH)。核磁共振碳谱(125 MHz, CD3CN):δ 14.1, 14.2(2×CH3),60.3, 60.6(2×CH2),93.9(O=CCC=O),117.6(2×ortho-ArC),125.1(para-ArC),130.1(2×meta-ArC),139.8(ArCquat-NH),151.9(NH-CH),165.6(C=O),168.8(氢键C=O)。
参考文献:
[1] Patent: WO2015/189560, 2015, A1. Location in patent: Page/Page column 87; 88
[2] Chemistry - A European Journal, 2010, vol. 16, # 9, p. 2938 - 2943
[3] Heterocycles, 2004, vol. 64, p. 177 - 191
[4] Tetrahedron, 1989, vol. 45, # 3, p. 889 - 908
[5] Bioorganic and Medicinal Chemistry Letters, 2006, vol. 16, # 3, p. 537 - 540
