13721-01-2
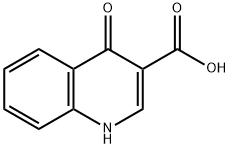 13721-01-2 结构式
13721-01-2 结构式
基本信息
VX-770中间体
4-二氢喹啉-3-羧酸
1,4-二氢-4-氧喹啉-3-甲酸
4-氧代-1,4-二氢3-喹啉羧酸
4-氧代-1,4-二氢喹啉-3-羧酸
4-氧代-1,4-二氢喹啉-3-甲酸
4-氧代-1,4-二氢喹啉-3-羧酸 1G
4-氧代-1,4-二氢喹啉-3-羧酸4-OXO-1
AURORA 17733
TIMTEC-BB SBB000272
VX770-INTERMEDIATES
4-Quinolone-3-carboxylic acid
4-Quinolone-3-carboxylic acid-D4
4-Oxo-1,4-dihydroquinoline Carboxylic Acid
4-Oxo-1,4-dihydroquinoline-3-carboxylicaci
1,4-Dihydro-4-oxo-3-quinolinecarboxylic acid
4-oxo-1,4-dihydro-3-quinolinecarboxylic acid
物理化学性质
制备方法
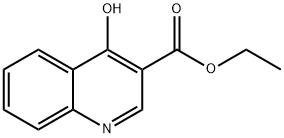
26892-90-0

13721-01-2
以4-羟基喹啉-3-甲酸乙酯为原料合成4-氧代-1,4-二氢喹啉-3-甲酸的一般步骤:将4-羟基喹啉-3-甲酸乙酯(15g,69mmol)悬浮于2N氢氧化钠溶液(150mL)中,于回流条件下搅拌反应2小时。反应完成后,将混合物冷却至室温,过滤除去不溶物。滤液用2N盐酸酸化至pH4,析出白色沉淀。过滤收集沉淀,用去离子水洗涤数次,最后在真空条件下干燥,得到4-氧代-1,4-二氢喹啉-3-甲酸,为浅白色固体(10.5g,收率92%)。产物经1H NMR(DMSO-d6)表征:δ15.34(s,1H),13.42(s,1H),8.89(s,1H),8.28(d,J=8.0Hz,1H),7.88(m,1H),7.81(d,J=8.4Hz,1H),7.60(m,1H)。
参考文献:
[1] Patent: US2008/90864, 2008, A1. Location in patent: Page/Page column 7
[2] Patent: WO2007/79139, 2007, A2. Location in patent: Page/Page column 46; 47
[3] Patent: US2012/309758, 2012, A1. Location in patent: Page/Page column 61
[4] Patent: US2015/231142, 2015, A1. Location in patent: Paragraph 0352
[5] Patent: WO2018/64632, 2018, A1. Location in patent: Paragraph 00215; 00216
| 报价日期 | 产品编号 | 产品名称 | CAS号 | 包装 | 价格 |
| 2025/12/22 | D4881 | 1,4-二氢-4-氧喹啉-3-甲酸 1,4-Dihydro-4-oxoquinoline-3-carboxylic Acid | 13721-01-2 | 1g | 60元 |
| 2025/12/22 | D4881 | 1,4-二氢-4-氧喹啉-3-甲酸 1,4-Dihydro-4-oxoquinoline-3-carboxylic Acid | 13721-01-2 | 5g | 230元 |
| 2025/05/22 | XW137210123 | 4-氧代-1,4-二氢喹啉-3-羧酸 1,4-dihydro-4-oxoquinoline-3-carboxylic acid;1,4-dihydro-4-oxo-3-quinolinecarboxylic acid;4-oxo-1,4-dihydroquinoline carboxylic acid | 13721-01-2 | 25G | 438元 |
